Abstract
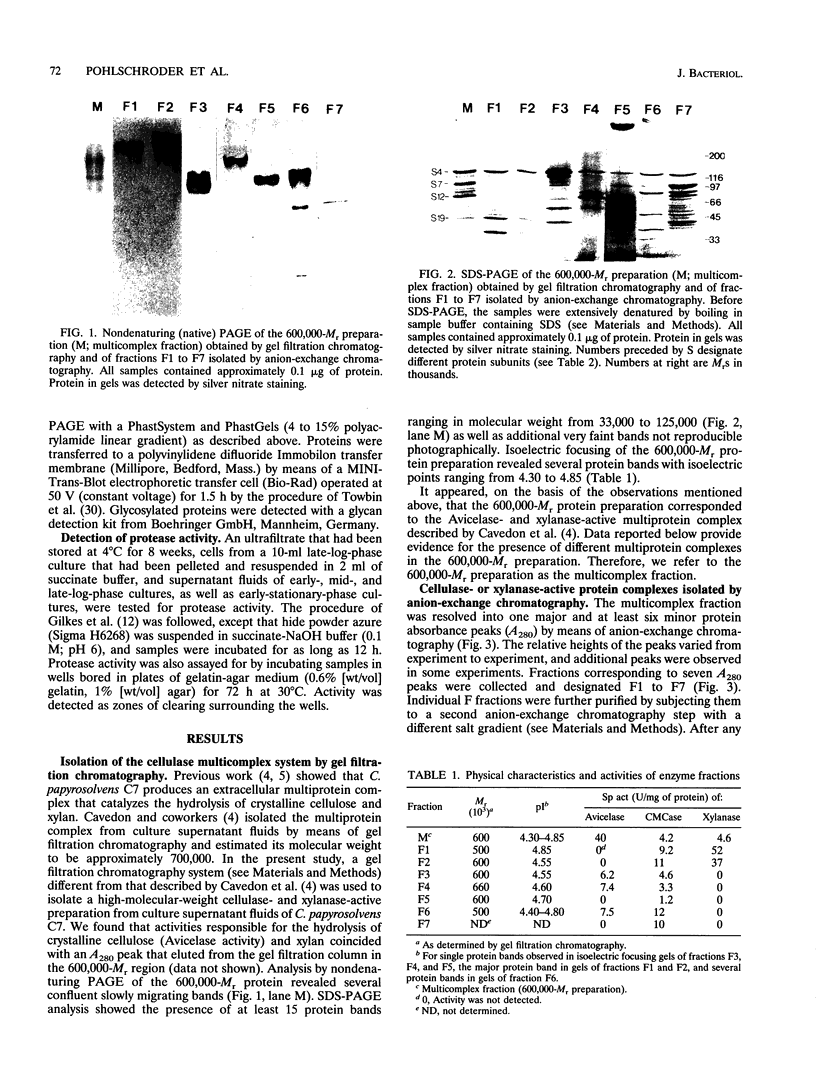
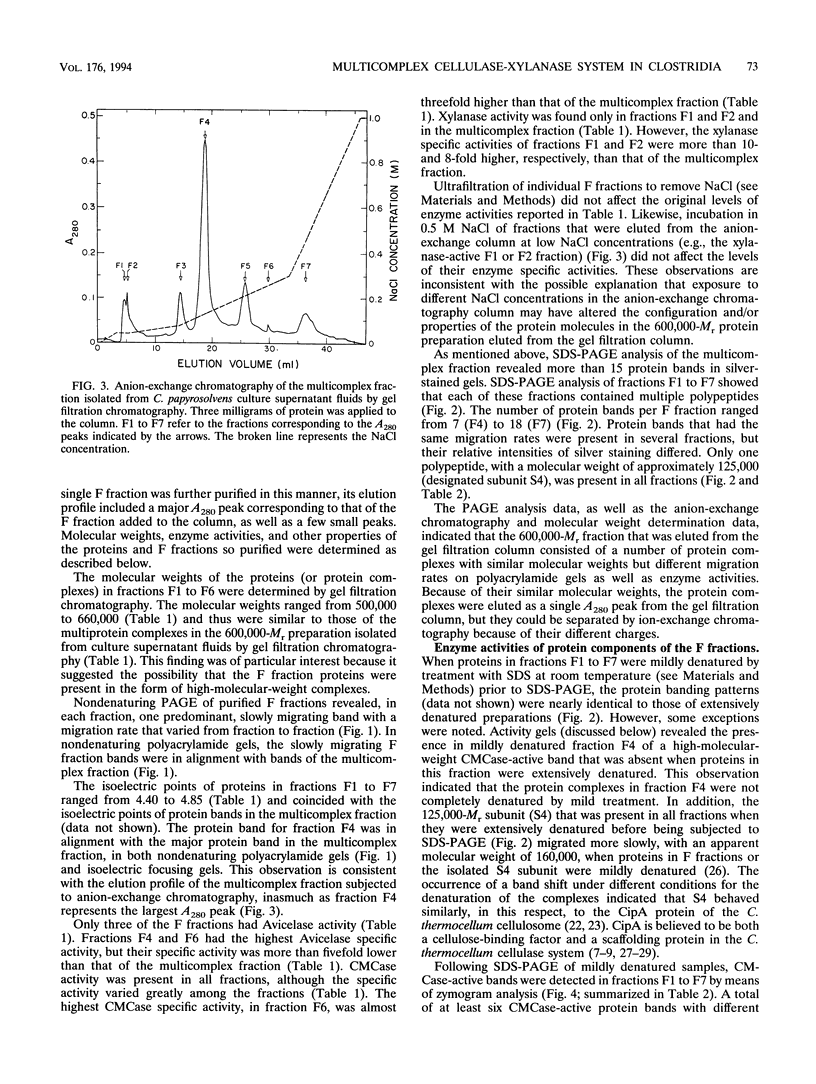
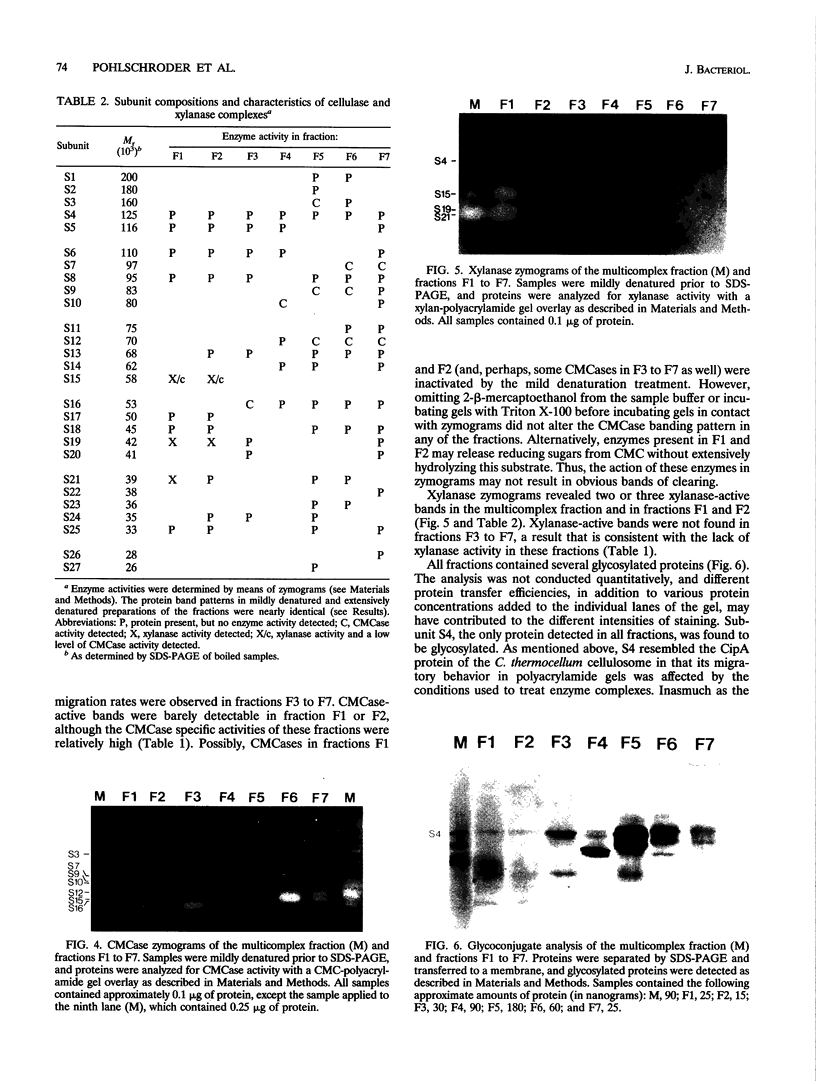
The cellulase system of Clostridium papyrosolvens C7 was fractionated by means of ion-exchange chromatography into at least seven high-molecular-weight multiprotein complexes, each with different enzymatic and structural properties. The molecular weights of the complexes, as determined by gel filtration chromatography, ranged from 500,000 to 660,000, and the isoelectric points ranged from 4.40 to 4.85. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the complexes showed that each complex had a distinct polypeptide composition. Avicelase, carboxymethyl cellulase, and xylanase activity profiles differed from protein complex to protein complex. Three of the complexes hydrolyzed crystalline cellulose (Avicel). Activity zymograms of gels (following electrophoresis under mildly denaturing conditions) revealed different carboxymethyl cellulase-active proteins in all complexes but xylanase-active proteins in only two of the complexes. The xylanase specific activity of these two complexes was more than eightfold higher than that of the unfractionated cellulase preparation. A 125,000-M(r) glycoprotein with no apparent enzyme activity was the only polypeptide present in all seven complexes. Experiments involving recombination of samples eluted from the ion-exchange chromatography column indicated that synergistic interactions occurred in the hydrolysis of crystalline cellulose by the cellulase system. We propose that the C. papyrosolvens enzyme system responsible for the hydrolysis of crystalline cellulose and xylan is a multicomplex system comprising at least seven diverse protein complexes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béguin P., Millet J., Aubert J. P. Cellulose degradation by Clostridium thermocellum: from manure to molecular biology. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):523–528. doi: 10.1111/j.1574-6968.1992.tb14087.x. [DOI] [PubMed] [Google Scholar]
- Béguin P., Millet J., Chauvaux S., Salamitou S., Tokatlidis K., Navas J., Fujino T., Lemaire M., Raynaud O., Daniel M. K. Bacterial cellulases. Biochem Soc Trans. 1992 Feb;20(1):42–46. doi: 10.1042/bst0200042. [DOI] [PubMed] [Google Scholar]
- Cavedon K., Leschine S. B., Canale-Parola E. Cellulase system of a free-living, mesophilic clostridium (strain C7). J Bacteriol. 1990 Aug;172(8):4222–4230. doi: 10.1128/jb.172.8.4222-4230.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedon K., Leschine S. B., Canale-Parola E. Characterization of the extracellular cellulase from a mesophilic clostridium (strain C7). J Bacteriol. 1990 Aug;172(8):4231–4237. doi: 10.1128/jb.172.8.4231-4237.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T., Béguin P., Aubert J. P. Cloning of a Clostridium thermocellum DNA fragment encoding polypeptides that bind the catalytic components of the cellulosome. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):165–170. doi: 10.1016/0378-1097(92)90602-k. [DOI] [PubMed] [Google Scholar]
- Fujino T., Béguin P., Aubert J. P. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J Bacteriol. 1993 Apr;175(7):1891–1899. doi: 10.1128/jb.175.7.1891-1899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerngross U. T., Romaniec M. P., Kobayashi T., Huskisson N. S., Demain A. L. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol Microbiol. 1993 Apr;8(2):325–334. doi: 10.1111/j.1365-2958.1993.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Gerwig G. J., de Waard P., Kamerling J. P., Vliegenthart J. F., Morgenstern E., Lamed R., Bayer E. A. Novel O-linked carbohydrate chains in the cellulase complex (cellulosome) of Clostridium thermocellum. 3-O-Methyl-N-acetylglucosamine as a constituent of a glycoprotein. J Biol Chem. 1989 Jan 15;264(2):1027–1035. [PubMed] [Google Scholar]
- Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring S., Wiegel J., Mayer F. Subunit Composition and Glycosidic Activities of the Cellulase Complex from Clostridium thermocellum JW20. Appl Environ Microbiol. 1990 Dec;56(12):3798–3804. doi: 10.1128/aem.56.12.3798-3804.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Mesophilic cellulolytic clostridia from freshwater environments. Appl Environ Microbiol. 1983 Sep;46(3):728–737. doi: 10.1128/aem.46.3.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag E., Bayer E. A., Lamed R. Anomalous dissociative behavior of the major glycosylated component of the cellulosome of Clostridium thermocellum. Appl Biochem Biotechnol. 1991 Aug;30(2):129–136. doi: 10.1007/BF02921680. [DOI] [PubMed] [Google Scholar]
- Poole D. M., Morag E., Lamed R., Bayer E. A., Hazlewood G. P., Gilbert H. J. Identification of the cellulose-binding domain of the cellulosome subunit S1 from Clostridium thermocellum YS. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):181–186. doi: 10.1016/0378-1097(92)90022-g. [DOI] [PubMed] [Google Scholar]
- Salamitou S., Tokatlidis K., Béguin P., Aubert J. P. Involvement of separate domains of the cellulosomal protein S1 of Clostridium thermocellum in binding to cellulose and in anchoring of catalytic subunits to the cellulosome. FEBS Lett. 1992 Jun 8;304(1):89–92. doi: 10.1016/0014-5793(92)80595-8. [DOI] [PubMed] [Google Scholar]
- Tokatlidis K., Salamitou S., Béguin P., Dhurjati P., Aubert J. P. Interaction of the duplicated segment carried by Clostridium thermocellum cellulases with cellulosome components. FEBS Lett. 1991 Oct 21;291(2):185–188. doi: 10.1016/0014-5793(91)81279-h. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. K., Kruus K., Wu J. H. Cloning and DNA sequence of the gene coding for Clostridium thermocellum cellulase Ss (CelS), a major cellulosome component. J Bacteriol. 1993 Mar;175(5):1293–1302. doi: 10.1128/jb.175.5.1293-1302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]