Abstract
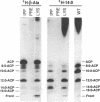
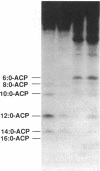
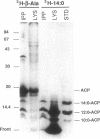
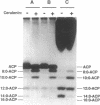
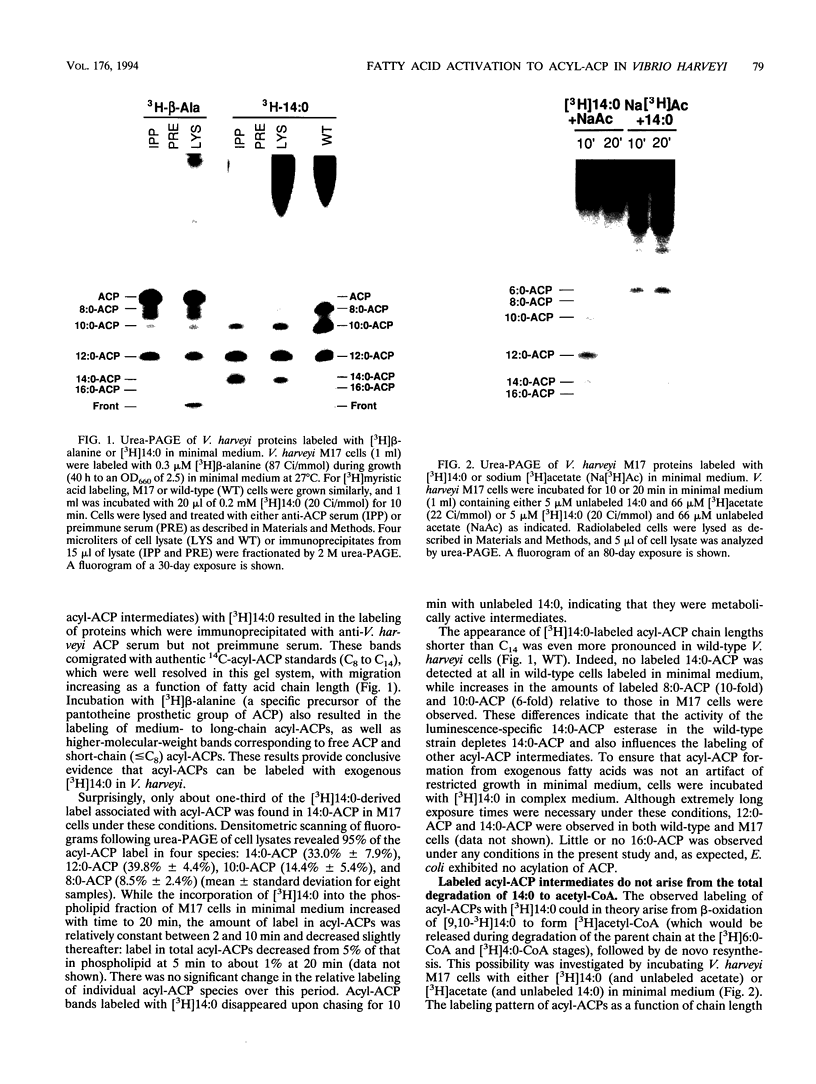
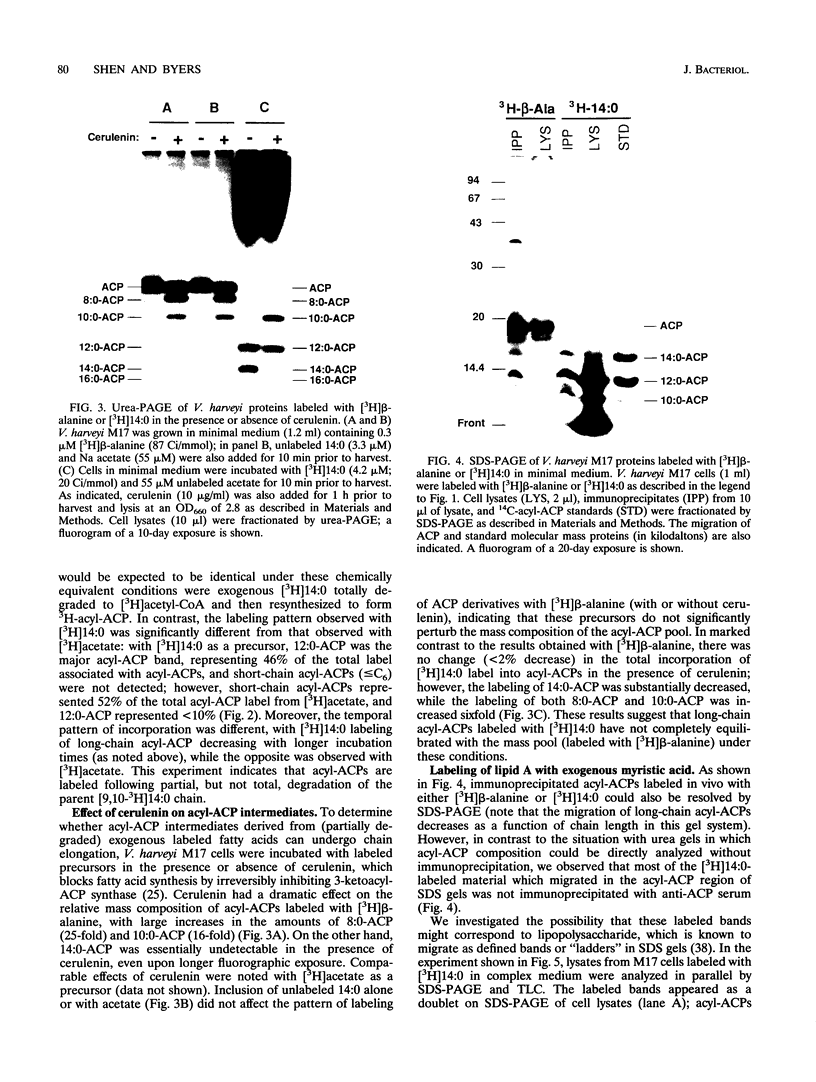
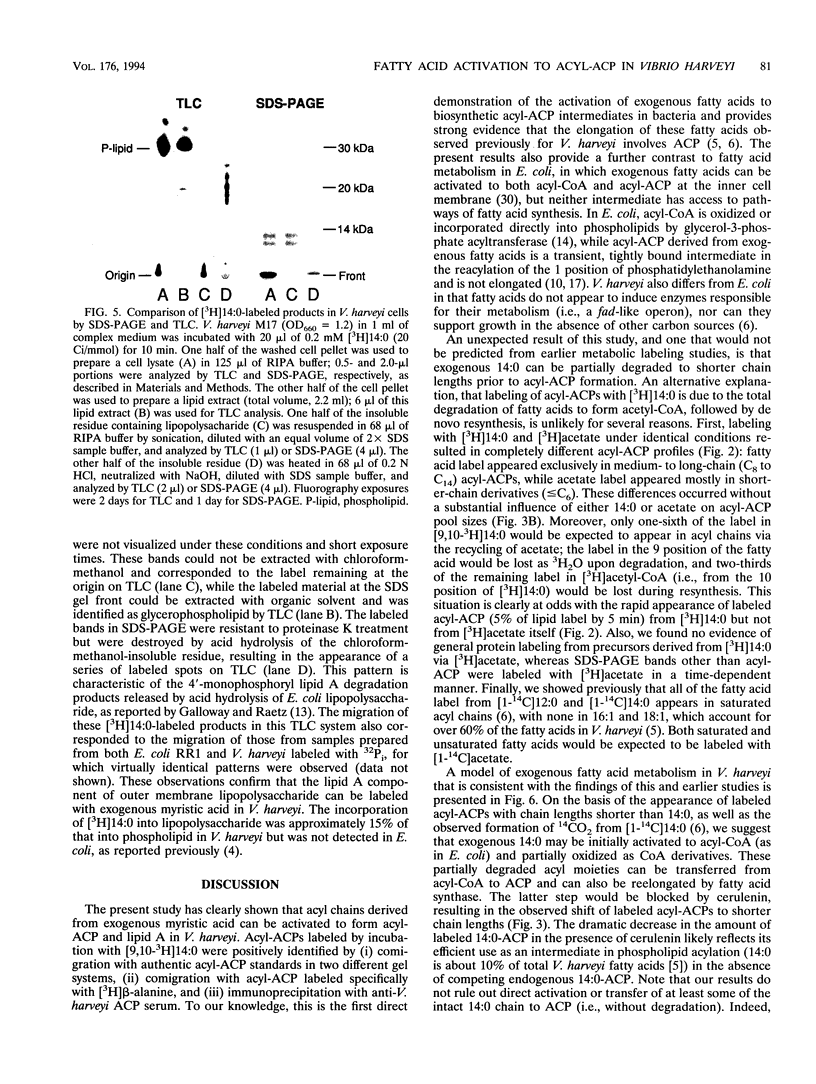
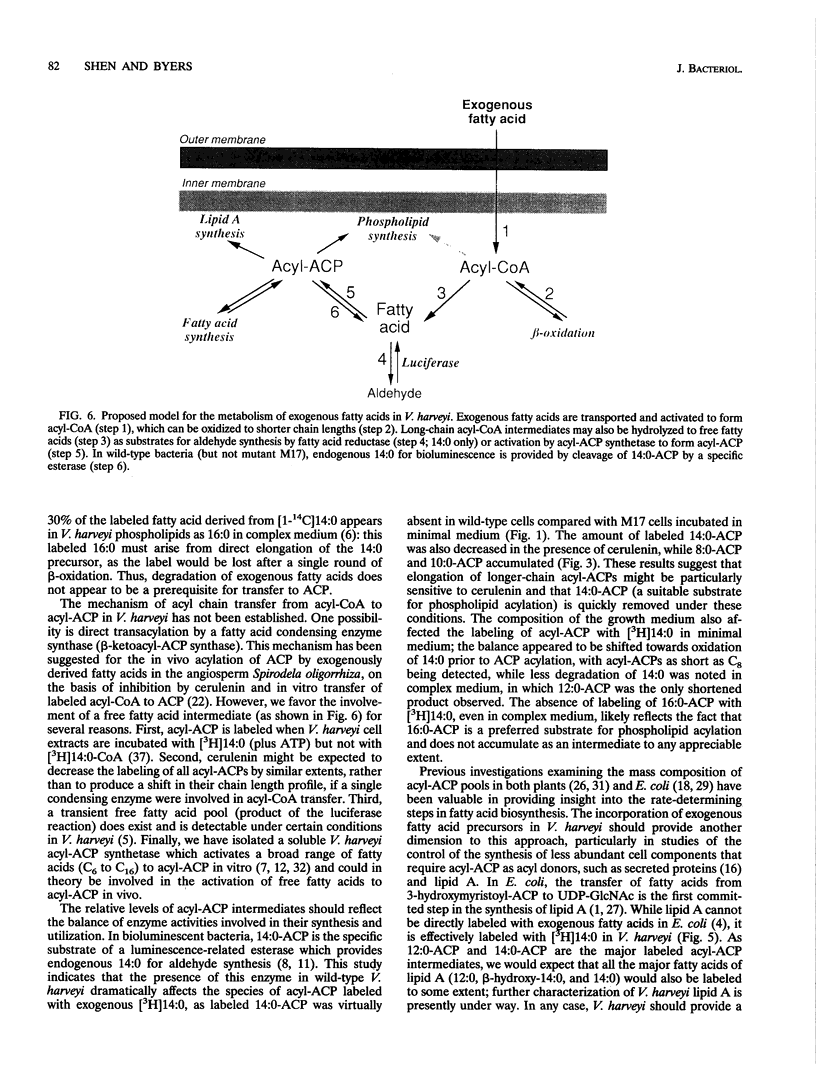
To study the involvement of acyl carrier protein (ACP) in the metabolism of exogenous fatty acids in Vibrio harveyi, cultures were incubated in minimal medium with [9,10-3H]myristic acid, and labeled proteins were analyzed by gel electrophoresis. Labeled acyl-ACP was positively identified by immunoprecipitation with anti-V. harveyi ACP serum and comigration with acyl-ACP standards and [3H]beta-alanine-labeled bands on both sodium dodecyl sulfate- and urea-polyacrylamide gels. Surprisingly, most of the acyl-ACP label corresponded to fatty acid chain lengths of less than 14 carbons: C14, C12, C10, and C8 represented 33, 40, 14, and 8% of total [3H]14:0-derived acyl-ACPs, respectively, in a dark mutant (M17) of V. harveyi which lacks myristoyl-ACP esterase activity; however, labeled 14:0-ACP was absent in the wild-type strain. 14:0- and 12:0-ACP were also the predominant species labeled in complex medium. In contrast, short-chain acyl-ACPs (< or = C6) were the major labeled derivatives when V. harveyi was incubated with [3H]acetate, indicating that acyl-ACP labeling with [3H]14:0 in vivo is not due to the total degradation of [3H]14:0 to [3H]acetyl coenzyme A followed by resynthesis. Cerulenin increased the mass of medium- to long-chain acyl-ACPs (> or = C8) labeled with [3H]beta-alanine fivefold, while total incorporation of [3H]14:0 was not affected, although a shift to shorter chain lengths was noted. Additional bands which comigrated with acyl-ACP on sodium dodecyl sulfate gels were identified as lipopolysaccharide by acid hydrolysis and thin-layer chromatography. The levels of incorporation of [3H] 14:0 into acyl-ACP and lipopolysaccharide were 2 and 15%, respectively, of that into phospholipid by 10 min. Our results indicate that in contrast to the situation in Escherichia coli, exogenous fatty acids can be activated to acyl-ACP intermediates after partial degradation in V. harveyi and can effectively label products (i.e., lipid A) that require ACP as an acyl donor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. S., Raetz C. R. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987 Apr 15;262(11):5159–5169. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Black P. N., Said B., Ghosn C. R., Beach J. V., Nunn W. D. Purification and characterization of an outer membrane-bound protein involved in long-chain fatty acid transport in Escherichia coli. J Biol Chem. 1987 Jan 25;262(3):1412–1419. [PubMed] [Google Scholar]
- Brozek K. A., Raetz C. R. Biosynthesis of lipid A in Escherichia coli. Acyl carrier protein-dependent incorporation of laurate and myristate. J Biol Chem. 1990 Sep 15;265(26):15410–15417. [PubMed] [Google Scholar]
- Byers D. M. Elongation of exogenous fatty acids by the bioluminescent bacterium Vibrio harveyi. J Bacteriol. 1989 Jan;171(1):59–64. doi: 10.1128/jb.171.1.59-64.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D. M., Holmes C. G. A soluble fatty acyl-acyl carrier protein synthetase from the bioluminescent bacterium Vibrio harveyi. Biochem Cell Biol. 1990 Jul-Aug;68(7-8):1045–1051. doi: 10.1139/o90-154. [DOI] [PubMed] [Google Scholar]
- Byers D. M., Meighen E. A. Acyl-acyl carrier protein as a source of fatty acids for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6085–6089. doi: 10.1073/pnas.82.18.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. L., Hsu L., Jackowski S., Rock C. O. 2-Acylglycerolphosphoethanolamine acyltransferase/acyl-acyl carrier protein synthetase is a membrane-associated acyl carrier protein binding protein. J Biol Chem. 1989 May 5;264(13):7384–7389. [PubMed] [Google Scholar]
- Ferri S. R., Meighen E. A. A lux-specific myristoyl transferase in luminescent bacteria related to eukaryotic serine esterases. J Biol Chem. 1991 Jul 15;266(20):12852–12857. [PubMed] [Google Scholar]
- Fice D., Shen Z., Byers D. M. Purification and characterization of fatty acyl-acyl carrier protein synthetase from Vibrio harveyi. J Bacteriol. 1993 Apr;175(7):1865–1870. doi: 10.1128/jb.175.7.1865-1870.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway S. M., Raetz C. R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990 Apr 15;265(11):6394–6402. [PubMed] [Google Scholar]
- Green P. R., Merrill A. H., Jr, Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Purification, reconstitution, and characterization of sn-glycerol-3-phosphate acyltransferase. J Biol Chem. 1981 Nov 10;256(21):11151–11159. [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Hsu L., Rock C. O. 2-acylglycerophosphoethanolamine acyltransferase/acyl-[acyl-carrier- protein] synthetase from Escherichia coli. Methods Enzymol. 1992;209:111–117. doi: 10.1016/0076-6879(92)09015-u. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Acetoacetyl-acyl carrier protein synthase, a potential regulator of fatty acid biosynthesis in bacteria. J Biol Chem. 1987 Jun 5;262(16):7927–7931. [PubMed] [Google Scholar]
- Kameda K., Nunn W. D. Purification and characterization of acyl coenzyme A synthetase from Escherichia coli. J Biol Chem. 1981 Jun 10;256(11):5702–5707. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Callahan F. E., Mehta R. A., Ohlrogge J. B. Rapid in Vivo Acylation of Acyl Carrier Protein with Exogenous Fatty Acids in Spirodela oligorrhiza. Plant Physiol. 1989 Feb;89(2):707–711. doi: 10.1104/pp.89.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C. M., Boylan M., Graham A. F., Meighen E. A. Organization of the lux structural genes of Vibrio harveyi. Expression under the T7 bacteriophage promoter, mRNA analysis, and nucleotide sequence of the luxD gene. J Biol Chem. 1988 Sep 15;263(26):13393–13399. [PubMed] [Google Scholar]
- Nunn W. D. A molecular view of fatty acid catabolism in Escherichia coli. Microbiol Rev. 1986 Jun;50(2):179–192. doi: 10.1128/mr.50.2.179-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. [PubMed] [Google Scholar]
- Post-Beittenmiller D., Jaworski J. G., Ohlrogge J. B. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991 Jan 25;266(3):1858–1865. [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Pathways for the incorporation of exogenous fatty acids into phosphatidylethanolamine in Escherichia coli. J Biol Chem. 1985 Oct 15;260(23):12720–12724. [PubMed] [Google Scholar]
- Rock C. O., Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982 Sep 25;257(18):10759–10765. [PubMed] [Google Scholar]
- Roughan G., Nishida I. Concentrations of long-chain acyl-acyl carrier proteins during fatty acid synthesis by chloroplasts isolated from pea (Pisum sativum), safflower (Carthamus tinctoris), and amaranthus (Amaranthus lividus) leaves. Arch Biochem Biophys. 1990 Jan;276(1):38–46. doi: 10.1016/0003-9861(90)90007-l. [DOI] [PubMed] [Google Scholar]
- Shen Z., Fice D., Byers D. M. Preparation of fatty-acylated derivatives of acyl carrier protein using Vibrio harveyi acyl-ACP synthetase. Anal Biochem. 1992 Jul;204(1):34–39. doi: 10.1016/0003-2697(92)90135-t. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Myristic acid stimulation of bacterial bioluminescence in "aldehyde" mutants. Proc Natl Acad Sci U S A. 1978 Jan;75(1):266–269. doi: 10.1073/pnas.75.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Boom T., Cronan J. E., Jr Genetics and regulation of bacterial lipid metabolism. Annu Rev Microbiol. 1989;43:317–343. doi: 10.1146/annurev.mi.43.100189.001533. [DOI] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., Rodriguez A., Meighen E. Intersubunit transfer of fatty acyl groups during fatty acid reduction. J Biol Chem. 1986 Dec 5;261(34):15981–15988. [PubMed] [Google Scholar]
- Yeh H. Y., Jacobs D. M. Characterization of lipopolysaccharide fractions and their interactions with cells and model membranes. J Bacteriol. 1992 Jan;174(1):336–341. doi: 10.1128/jb.174.1.336-341.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]