Abstract
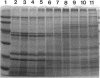
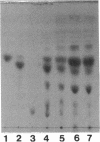
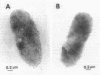
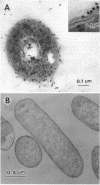
From the cell envelope preparation of Sphingomonas paucimobilis two membrane fractions with different densities were separated by sucrose density gradient ultracentrifugation. The high-density fraction contained several major proteins, phospholipids, and glycosphingolipids, which are the only glycolipids of this lipopolysaccharide-lacking gram-negative bacterium. The low-density fraction showed many minor bands of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and NADH oxidase activity was localized in this fraction. Combined with morphological data of vesicles formed by these membrane fractions, the high-density and low-density fractions were proposed to be an outer membrane and a cytoplasmic membrane, respectively. The localization of the glycosphingolipid was investigated also by means of immunoelectron microscopic analysis using a glycosphingolipid-specific antibody. The glycosphingolipid was shown to localize at the cell envelope, and the antigenic sugar portion was exposed to the bacterial cell surface. From these results the glycosphingolipid was assumed to have a function similar to that of the lipopolysaccharide of other gram-negative bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloeckaert A., Zygmunt M. S., Nicolle J. C., Dubray G., Limet J. N. O-chain expression in the rough Brucella melitensis strain B115: induction of O-polysaccharide-specific monoclonal antibodies and intracellular localization demonstrated by immunoelectron microscopy. J Gen Microbiol. 1992 Jun;138(6):1211–1219. doi: 10.1099/00221287-138-6-1211. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Suzuki T., Suzuki Y., Taki T., Matsumoto M., Higashi H., Kato S. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J Biochem. 1983 Jul;94(1):327–330. doi: 10.1093/oxfordjournals.jbchem.a134350. [DOI] [PubMed] [Google Scholar]
- Kastowsky M., Gutberlet T., Bradaczek H. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol. 1992 Jul;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K., Seydel U., Matsuura M., Danbara H., Rietschel E. T., Zähringer U. Chemical structure of glycosphingolipids isolated from Sphingomonas paucimobilis. FEBS Lett. 1991 Nov 4;292(1-2):107–110. doi: 10.1016/0014-5793(91)80845-t. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Uchida K., Aida K. Isolation of an unusual 'lipid A' type glycolipid from Pseudomonas paucimobilis. Biochim Biophys Acta. 1982 Sep 14;712(3):571–575. doi: 10.1016/0005-2760(82)90285-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi E., Yano I., Oyaizu H., Hashimoto Y., Ezaki T., Yamamoto H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol Immunol. 1990;34(2):99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Tokuda H., Mizushima S. Proton motive force-dependent and -independent protein translocation revealed by an efficient in vitro assay system of Escherichia coli. J Biol Chem. 1989 Jan 25;264(3):1723–1728. [PubMed] [Google Scholar]