Abstract
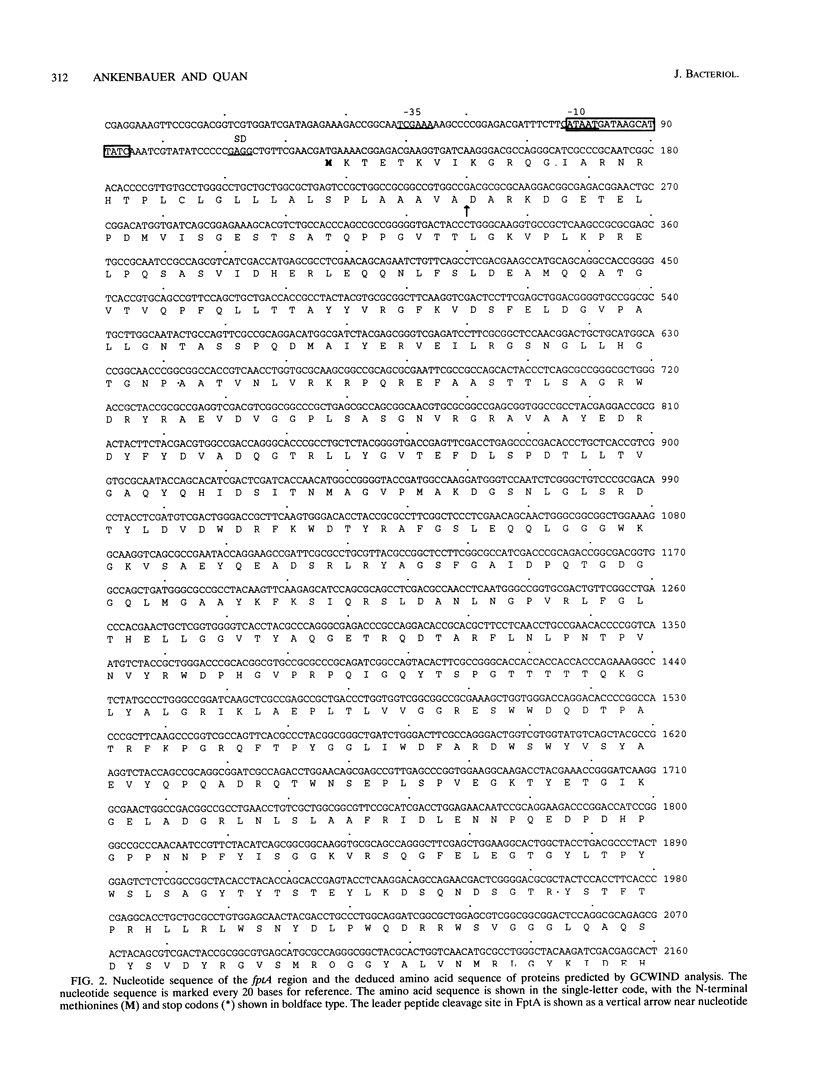
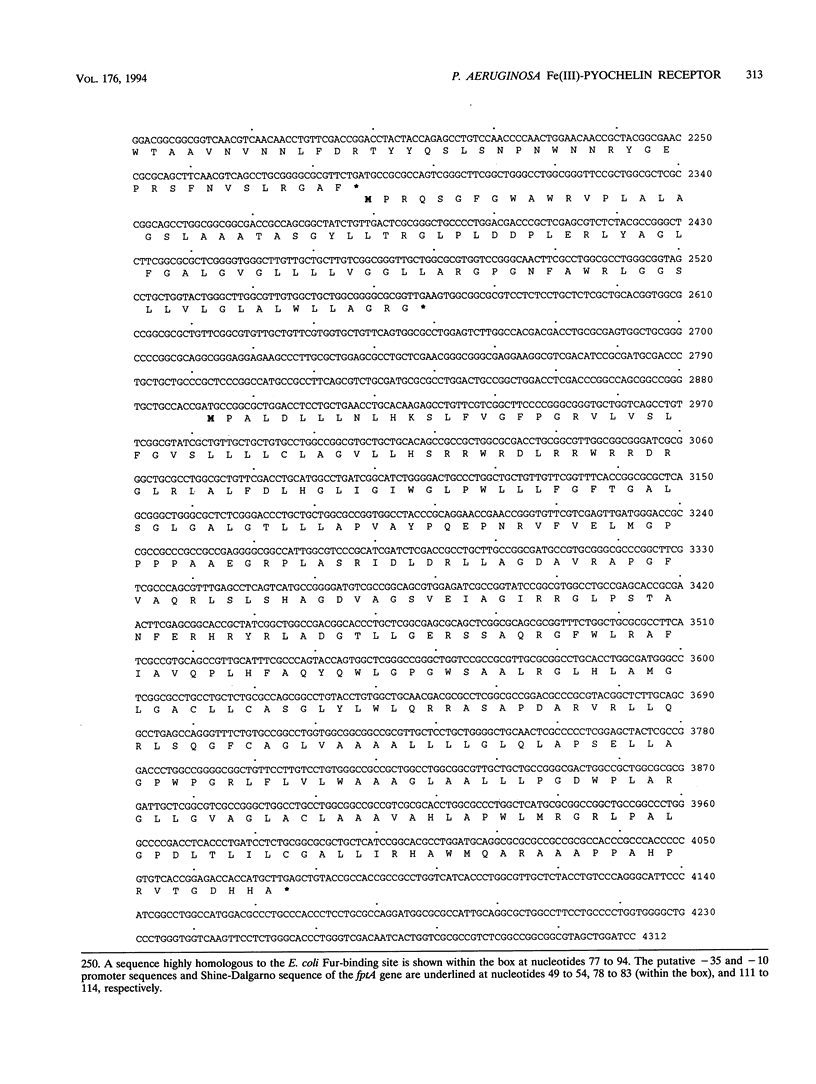
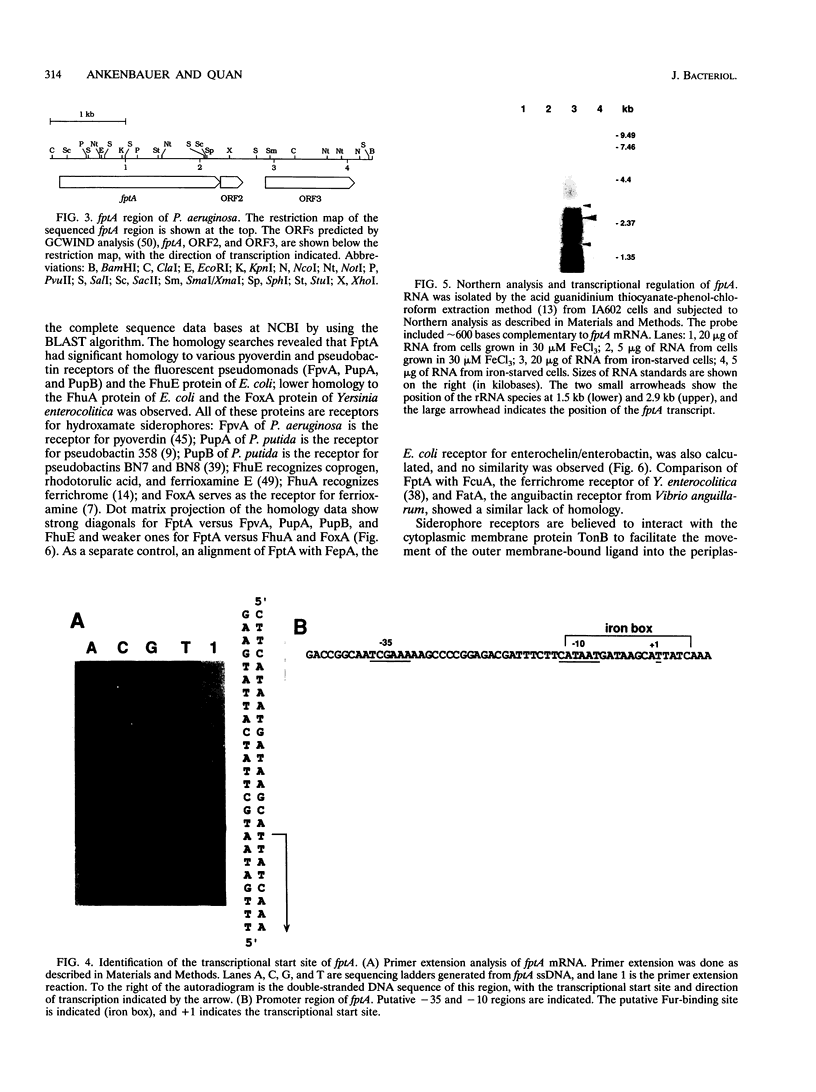
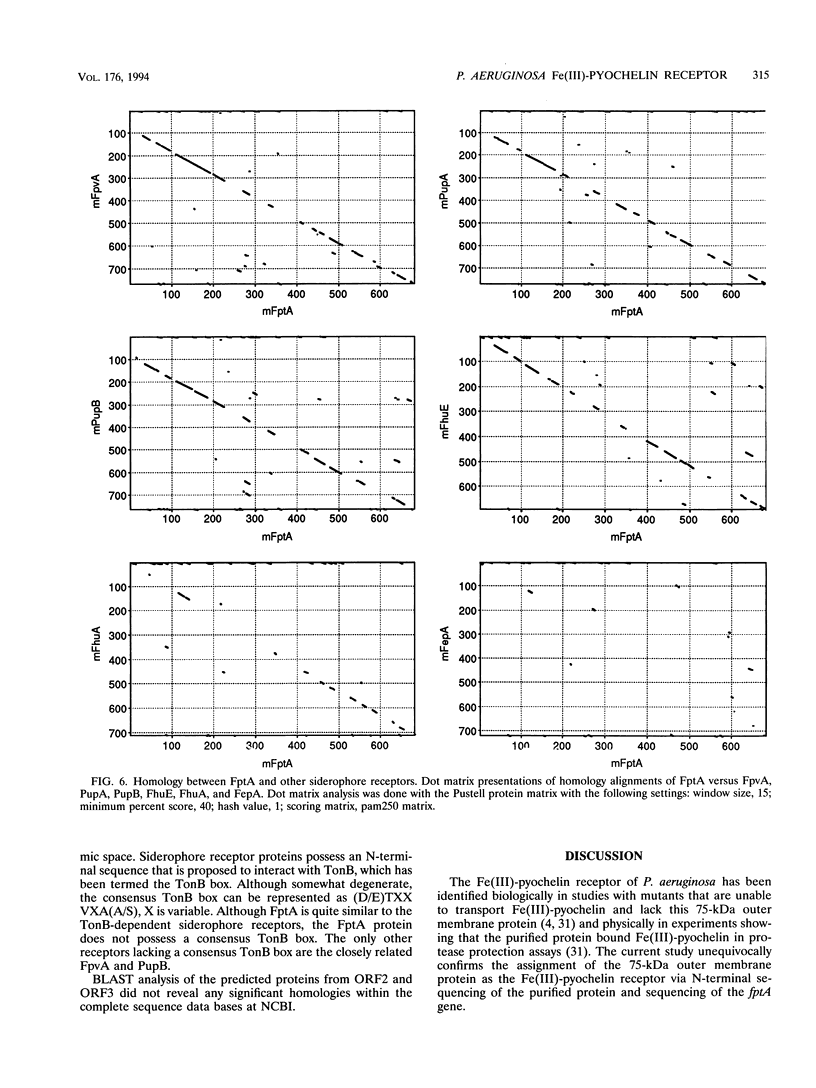
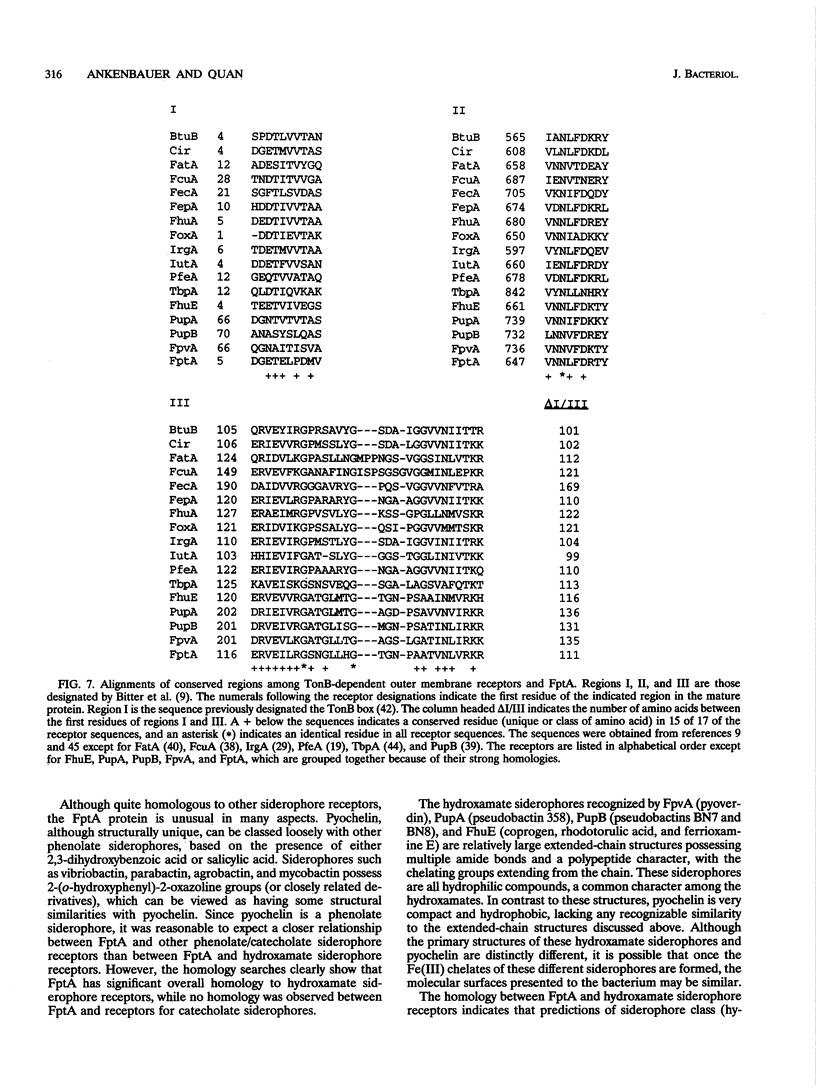
The Pseudomonas aeruginosa siderophore pyochelin is structurally unique among siderophores and possesses neither hydroxamate- nor catecholate-chelating groups. The structural gene encoding the 75-kDa outer membrane Fe(III)-pyochelin receptor FptA has been isolated by plasmid rescue techniques and sequenced. The N-terminal amino acid sequence of the isolated FptA protein corresponded to that deduced from the nucleotide sequence of the fptA structural gene. The mature FptA protein has 682 amino acids and a molecular mass of 75,993 Da and has considerable overall homology with the hydroxamate siderophore receptors FpvA of P. aeruginosa, PupA and PupB of Pseudomonas putida, and FhuE of Escherichia coli. This observation indicates that homologies between siderophore receptors are an unreliable predictor of siderophore ligand class recognition by a given receptor. The fptA gene was strongly regulated by iron; fptA transcription was totally repressed by 30 microM FeCl3, as determined by Northern (RNA) blotting. The promoter of the fptA gene contained the sequence 5'-ATAATGATAAGCATTATC-3', which matches the consensus E. coli Fur-binding site at 17 of 18 positions. The -10 promoter region and transcriptional start site of the fptA gene reside within this Fur-binding site.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R. G. Cloning of the outer membrane high-affinity Fe(III)-pyochelin receptor of Pseudomonas aeruginosa. J Bacteriol. 1992 Jul;174(13):4401–4409. doi: 10.1128/jb.174.13.4401-4409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R. G., Toyokuni T., Staley A., Rinehart K. L., Jr, Cox C. D. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol. 1988 Nov;170(11):5344–5351. doi: 10.1128/jb.170.11.5344-5351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R., Hanne L. F., Cox C. D. Mapping of mutations in Pseudomonas aeruginosa defective in pyoverdin production. J Bacteriol. 1986 Jul;167(1):7–11. doi: 10.1128/jb.167.1.7-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bitter W., Marugg J. D., de Weger L. A., Tommassen J., Weisbeek P. J. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol Microbiol. 1991 Mar;5(3):647–655. doi: 10.1111/j.1365-2958.1991.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Brickman T. J., Ozenberger B. A., McIntosh M. A. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990 Apr 20;212(4):669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Hantke K. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol Microbiol. 1992 May;6(10):1309–1321. doi: 10.1111/j.1365-2958.1992.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cornelissen C. N., Biswas G. D., Tsai J., Paruchuri D. K., Thompson S. A., Sparling P. F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992 Sep;174(18):5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Poole K. Cloning and characterization of the ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J Bacteriol. 1993 Jan;175(2):317–324. doi: 10.1128/jb.175.2.317-324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver J. M., Bryan L. E., Sokol P. A. Transformation of Pseudomonas aeruginosa by electroporation. Anal Biochem. 1990 Aug 15;189(1):75–79. doi: 10.1016/0003-2697(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Farinha M. A., Ronald S. L., Kropinski A. M., Paranchych W. Localization of the virulence-associated genes pilA, pilR, rpoN, fliA, fliC, ent, and fbp on the physical map of Pseudomonas aeruginosa PAO1 by pulsed-field electrophoresis. Infect Immun. 1993 Apr;61(4):1571–1575. doi: 10.1128/iai.61.4.1571-1575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A. E., Crosa J. H. Co-operative autoregulation of a replication protein gene. Mol Microbiol. 1991 Dec;5(12):3015–3023. doi: 10.1111/j.1365-2958.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Butterton J. R., Stoebner J. A., Payne S. M., Calderwood S. B. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol Microbiol. 1992 Aug;6(16):2407–2418. doi: 10.1111/j.1365-2958.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs D. E., Poole K. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J Bacteriol. 1993 Sep;175(18):5882–5889. doi: 10.1128/jb.175.18.5882-5889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs D. E., Young L., Poole K. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect Immun. 1991 Oct;59(10):3680–3684. doi: 10.1128/iai.59.10.3680-3684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Morgan A. F. Genome organization in Pseudomonas. Annu Rev Microbiol. 1986;40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Jones M. J. Simplified filter paper sandwich blot provides rapid, background-free northern blots. Biotechniques. 1992 May;12(5):684–688. [PubMed] [Google Scholar]
- Kamoun S., Tola E., Kamdar H., Kado C. I. Rapid generation of directed and unmarked deletions in Xanthomonas. Mol Microbiol. 1992 Mar;6(6):809–816. doi: 10.1111/j.1365-2958.1992.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Koebnik R., Hantke K., Braun V. The TonB-dependent ferrichrome receptor FcuA of Yersinia enterocolitica: evidence against a strict co-evolution of receptor structure and substrate specificity. Mol Microbiol. 1993 Feb;7(3):383–393. doi: 10.1111/j.1365-2958.1993.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Koster M., van de Vossenberg J., Leong J., Weisbeek P. J. Identification and characterization of the pupB gene encoding an inducible ferric-pseudobactin receptor of Pseudomonas putida WCS358. Mol Microbiol. 1993 May;8(3):591–601. doi: 10.1111/j.1365-2958.1993.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köster W. L., Actis L. A., Waldbeser L. S., Tolmasky M. E., Crosa J. H. Molecular characterization of the iron transport system mediated by the pJM1 plasmid in Vibrio anguillarum 775. J Biol Chem. 1991 Dec 15;266(35):23829–23833. [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Miller P. J., Wenzel R. P. Etiologic organisms as independent predictors of death and morbidity associated with bloodstream infections. J Infect Dis. 1987 Sep;156(3):471–477. doi: 10.1093/infdis/156.3.471. [DOI] [PubMed] [Google Scholar]
- Poole K., Neshat S., Krebes K., Heinrichs D. E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol. 1993 Aug;175(15):4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. W., Cox C. D., Vasil M. L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993 May;175(9):2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. W., Storey D. G., Vasil A. I., Vasil M. L. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PA01. Mol Microbiol. 1991 Nov;5(11):2823–2831. doi: 10.1111/j.1365-2958.1991.tb01991.x. [DOI] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol. 1990 Mar;4(3):427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Shields D. C., Higgins D. G., Sharp P. M. GCWIND: a microcomputer program for identifying open reading frames according to codon positional G+C content. Comput Appl Biosci. 1992 Oct;8(5):521–523. doi: 10.1093/bioinformatics/8.5.521. [DOI] [PubMed] [Google Scholar]
- Sokol P. A. Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia. J Clin Microbiol. 1986 Mar;23(3):560–562. doi: 10.1128/jcm.23.3.560-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A. Tn5 insertion mutants of Pseudomonas aeruginosa deficient in surface expression of ferripyochelin-binding protein. J Bacteriol. 1987 Jul;169(7):3365–3368. doi: 10.1128/jb.169.7.3365-3368.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Strom A. D., Hirst R., Petering J., Morgan A. Isolation of high frequency of recombination donors from Tn5 chromosomal mutants of Pseudomonas putida PPN and recalibration of the genetic map. Genetics. 1990 Nov;126(3):497–503. doi: 10.1093/genetics/126.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvé M., Moons M., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991 Mar 5;218(1):141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Teintze M., Hossain M. B., Barnes C. L., Leong J., van der Helm D. Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry. 1981 Oct 27;20(22):6446–6457. doi: 10.1021/bi00525a025. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Lara J. C., Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990 Jan;172(1):389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991 Apr;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- Visca P., Serino L., Orsi N. Isolation and characterization of Pseudomonas aeruginosa mutants blocked in the synthesis of pyoverdin. J Bacteriol. 1992 Sep;174(17):5727–5731. doi: 10.1128/jb.174.17.5727-5731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]