Abstract
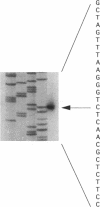
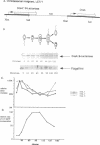
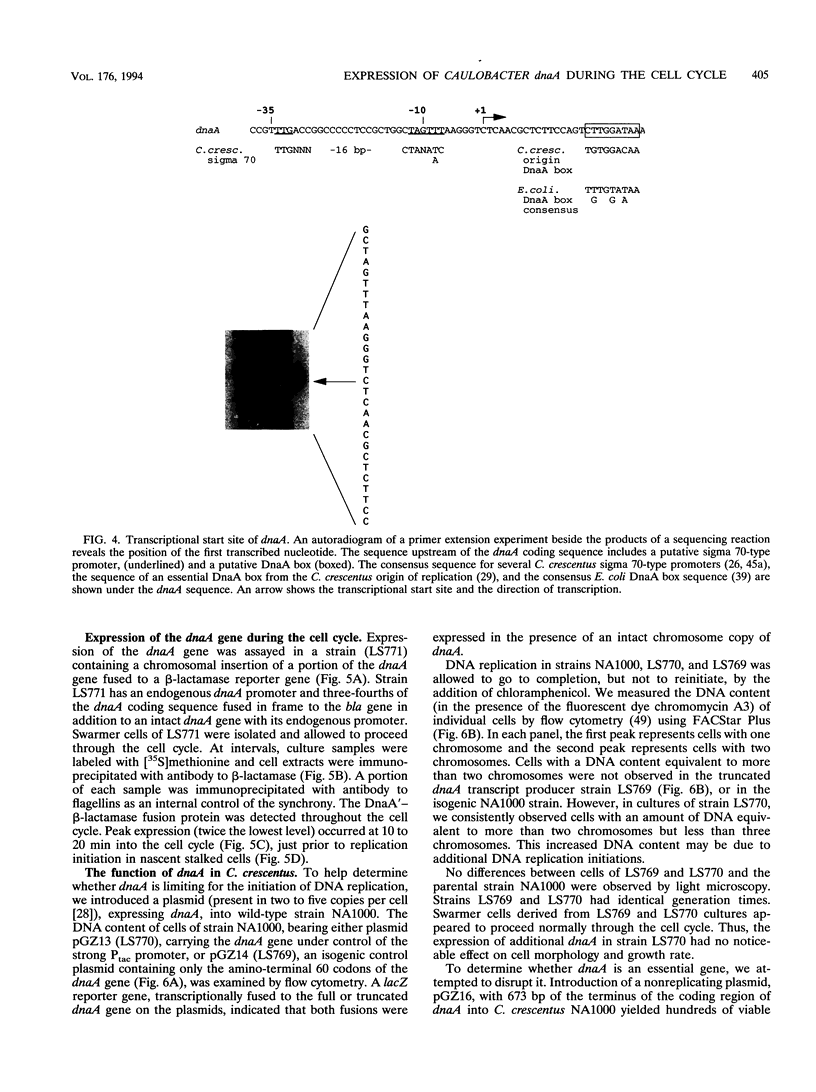
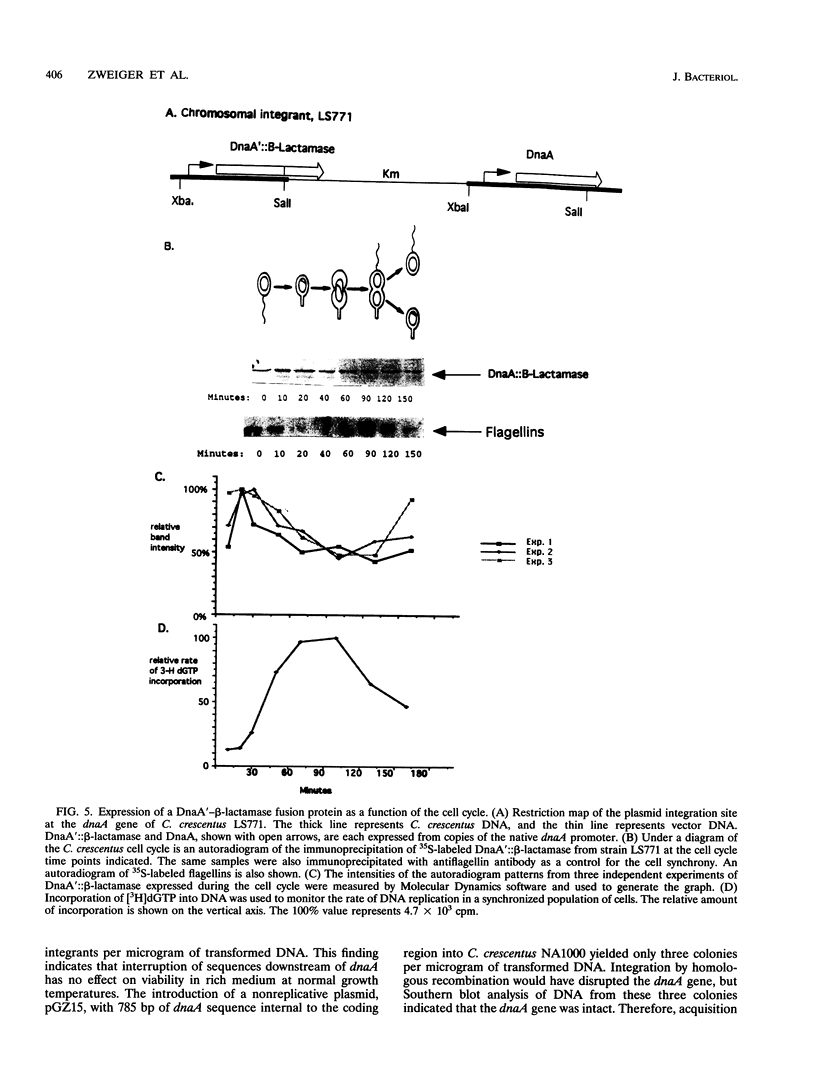
The initiation of DNA replication is under differential control in Caulobacter crescentus. Following cell division, only the chromosome in the progeny stalked cell is able to initiate DNA replication, while the chromosome in the progeny swarmer cell does not replicate until later in the cell cycle. We have isolated the dnaA gene in order to determine whether this essential and ubiquitous replication initiation protein also contributes to differential replication control in C. crescentus. Analysis of the cloned C. crescentus dnaA gene has shown that the deduced amino acid sequence can encode a 486-amino-acid protein that is 37% identical to the DnaA protein of Escherichia coli. The gene is located 2 kb from the origin of replication. Primer extension analysis revealed a single transcript originating from a sigma 70-type promoter. Immunoprecipitation of a DnaA'-beta-lactamase fusion protein showed that although expression occurs throughout the cell cycle, there is a doubling in the rate of expression just prior to the initiation of replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Clausen E. S., Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200(3):442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Churchward G., Holmans P., Bremer H. Increased expression of the dnaA gene has no effect on DNA replication in a dnaA+ strain of Escherichia coli. Mol Gen Genet. 1983;192(3):506–508. doi: 10.1007/BF00392197. [DOI] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972 Mar 14;64(3):671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall A., Shapiro L. Rate, origin, and bidirectionality of Caulobacter chromosome replication as determined by pulsed-field gel electrophoresis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):119–123. doi: 10.1073/pnas.86.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle H., Van de Merwe W., Madden K., Kampo G., Wright L., Donlon K. The nature of an intragenic suppressor of the Escherichia coli dnaA508 temperature-sensitive mutation. Gene. 1989 Dec 14;84(2):237–245. doi: 10.1016/0378-1119(89)90497-6. [DOI] [PubMed] [Google Scholar]
- Ely B. DNA sequence of the 3' end of the Caulobacter crescentus 16S rRNA gene. Nucleic Acids Res. 1992 Mar 25;20(6):1423–1423. doi: 10.1093/nar/20.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Johnson R. C. Generalized Transduction in CAULOBACTER CRESCENTUS. Genetics. 1977 Nov;87(3):391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger M., Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977 Oct;132(1):294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA and DnaA-box region in the Mycoplasma capricolum chromosome: conservation and variations in the course of evolution. Gene. 1992 Jan 2;110(1):17–23. doi: 10.1016/0378-1119(92)90439-v. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Micrococcus luteus: conservation and variations among eubacteria. Gene. 1990 Sep 1;93(1):73–78. doi: 10.1016/0378-1119(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Pseudomonas putida: conservation among three bacteria, Bacillus subtilis, Escherichia coli and P. putida. Mol Gen Genet. 1989 Feb;215(3):381–387. doi: 10.1007/BF00427033. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Baker T. A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J Biol Chem. 1987 Jul 25;262(21):10327–10334. [PubMed] [Google Scholar]
- Gomes S. L., Gober J. W., Shapiro L. Expression of the Caulobacter heat shock gene dnaK is developmentally controlled during growth at normal temperatures. J Bacteriol. 1990 Jun;172(6):3051–3059. doi: 10.1128/jb.172.6.3051-3059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., von Meyenburg K. Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet. 1979 Sep;175(2):135–144. doi: 10.1007/BF00425529. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hodgson D., Shaw P., O'Connell M., Henry S., Shapiro L. Caulobacter crescentus fatty acid-dependent cell cycle mutant. J Bacteriol. 1984 Apr;158(1):156–162. doi: 10.1128/jb.158.1.156-162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D. S., Crooke E., Kornberg A. Aggregated dnaA protein is dissociated and activated for DNA replication by phospholipase or dnaK protein. J Biol Chem. 1990 Nov 5;265(31):19244–19248. [PubMed] [Google Scholar]
- Kröger M., Wahl R., Schachtel G., Rice P. Compilation of DNA sequences of Escherichia coli (update 1992). Nucleic Acids Res. 1992 May 11;20 (Suppl):2119–2144. doi: 10.1093/nar/20.suppl.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Nucleotide sequence of the gene for ribosomal protein S20 and its flanking regions. J Biol Chem. 1981 Aug 10;256(15):8177–8182. [PubMed] [Google Scholar]
- Mansour J. D., Henry S., Shapiro L. Differential membrane phospholipid synthesis during the cell cycle of Caulobacter crescentus. J Bacteriol. 1980 Jan;141(1):262–269. doi: 10.1128/jb.141.1.262-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczynski G. T., Dingwall A., Shapiro L. Plasmid and chromosomal DNA replication and partitioning during the Caulobacter crescentus cell cycle. J Mol Biol. 1990 Apr 20;212(4):709–722. doi: 10.1016/0022-2836(90)90232-B. [DOI] [PubMed] [Google Scholar]
- Marczynski G. T., Shapiro L. Cell-cycle control of a cloned chromosomal origin of replication from Caulobacter crescentus. J Mol Biol. 1992 Aug 20;226(4):959–977. doi: 10.1016/0022-2836(92)91045-q. [DOI] [PubMed] [Google Scholar]
- O'Neill E. A., Bender R. A. Periodic synthesis of phospholipids during the Caulobacter crescentus cell cycle. J Bacteriol. 1987 Jun;169(6):2618–2623. doi: 10.1128/jb.169.6.2618-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Masurekar M., Newton A. Cloning and cell cycle-dependent expression of DNA replication gene dnaC from Caulobacter crescentus. J Bacteriol. 1990 Dec;172(12):7027–7034. doi: 10.1128/jb.172.12.7027-7034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Rickert M., Helmstetter C. E. DnaA protein overproduction abolishes cell cycle specificity of DNA replication from oriC in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3760–3766. doi: 10.1128/jb.171.7.3760-3766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaczek P., Wright A. Regulation of expression of the dnaA gene in Escherichia coli: role of the two promoters and the DnaA box. New Biol. 1990 Jun;2(6):574–582. [PubMed] [Google Scholar]
- Ream L. W., Margossian L., Clark A. J., Hansen F. G., von Meyenburg K. Genetic and physical mapping of recF in Escherichia coli K-12. Mol Gen Genet. 1980;180(1):115–121. doi: 10.1007/BF00267359. [DOI] [PubMed] [Google Scholar]
- Rizzo M. F., Shapiro L., Gober J. Asymmetric expression of the gyrase B gene from the replication-competent chromosome in the Caulobacter crescentus predivisional cell. J Bacteriol. 1993 Nov;175(21):6970–6981. doi: 10.1128/jb.175.21.6970-6981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Mizukami T. A temperature-sensitive Escherichia coli mutant defective in DNA replication: dnaN, a new gene adjacent to the dnaA gene. Mol Gen Genet. 1980;178(3):541–553. doi: 10.1007/BF00337859. [DOI] [PubMed] [Google Scholar]
- Schaefer C., Messer W. DnaA protein/DNA interaction. Modulation of the recognition sequence. Mol Gen Genet. 1991 Apr;226(1-2):34–40. doi: 10.1007/BF00273584. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987 Jul 17;50(2):259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Kornberg A. Cardiolipin activation of dnaA protein, the initiation protein of replication in Escherichia coli. J Biol Chem. 1988 May 25;263(15):7131–7135. [PubMed] [Google Scholar]
- Sekimizu K., Yung B. Y., Kornberg A. The dnaA protein of Escherichia coli. Abundance, improved purification, and membrane binding. J Biol Chem. 1988 May 25;263(15):7136–7140. [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfa-Selase F., Drabble W. T. Regulation of the gua operon of Escherichia coli by the DnaA protein. Mol Gen Genet. 1992 Jan;231(2):256–264. doi: 10.1007/BF00279799. [DOI] [PubMed] [Google Scholar]
- Yung B. Y., Kornberg A. Membrane attachment activates dnaA protein, the initiation protein of chromosome replication in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7202–7205. doi: 10.1073/pnas.85.19.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E. A., Geerse R. H., Memelink J., Bovenberg R. A., Magnée F. A., van de Putte P. Analysis of regulatory sequences upstream of the E. coli uvrB gene; involvement of the DnaA protein. Nucleic Acids Res. 1985 Mar 25;13(6):1829–1840. doi: 10.1093/nar/13.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]