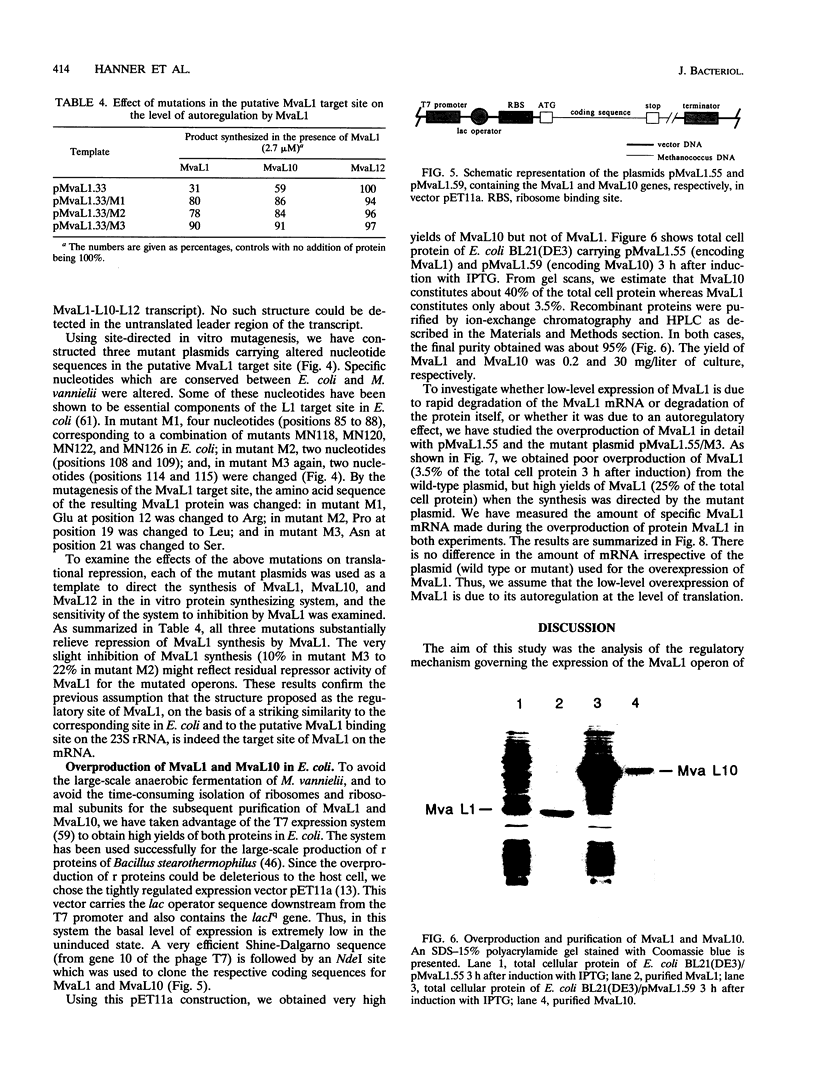
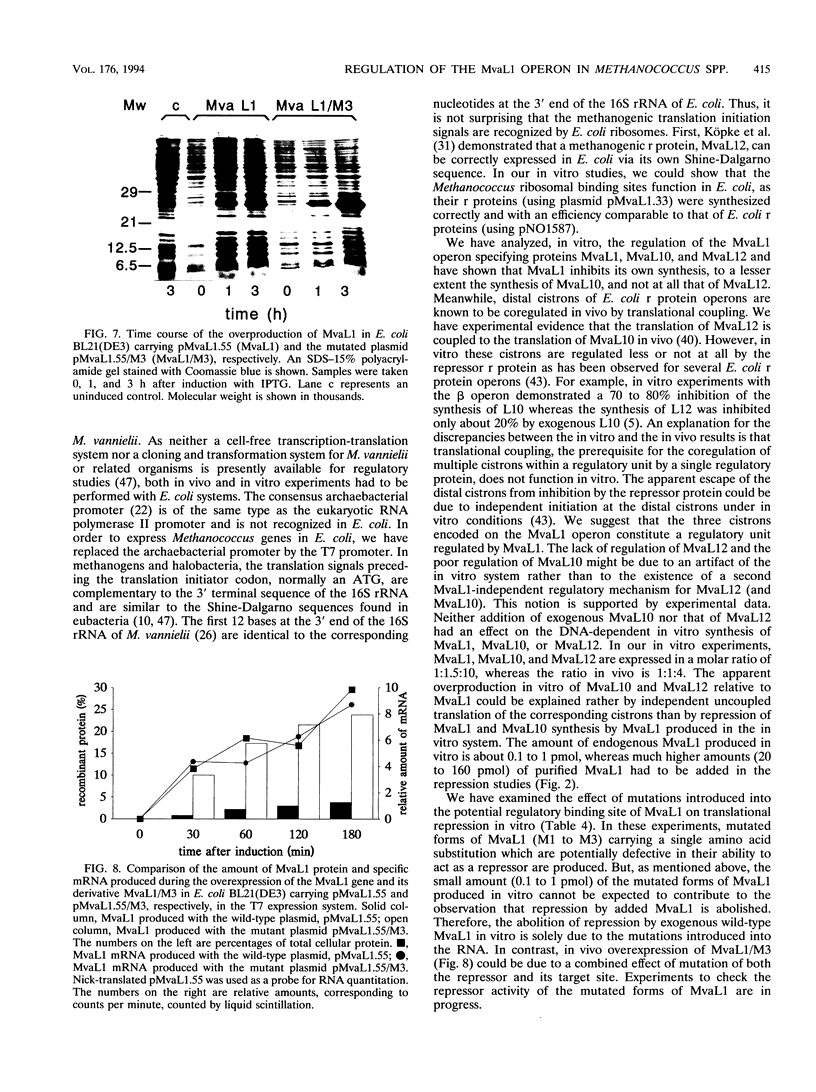
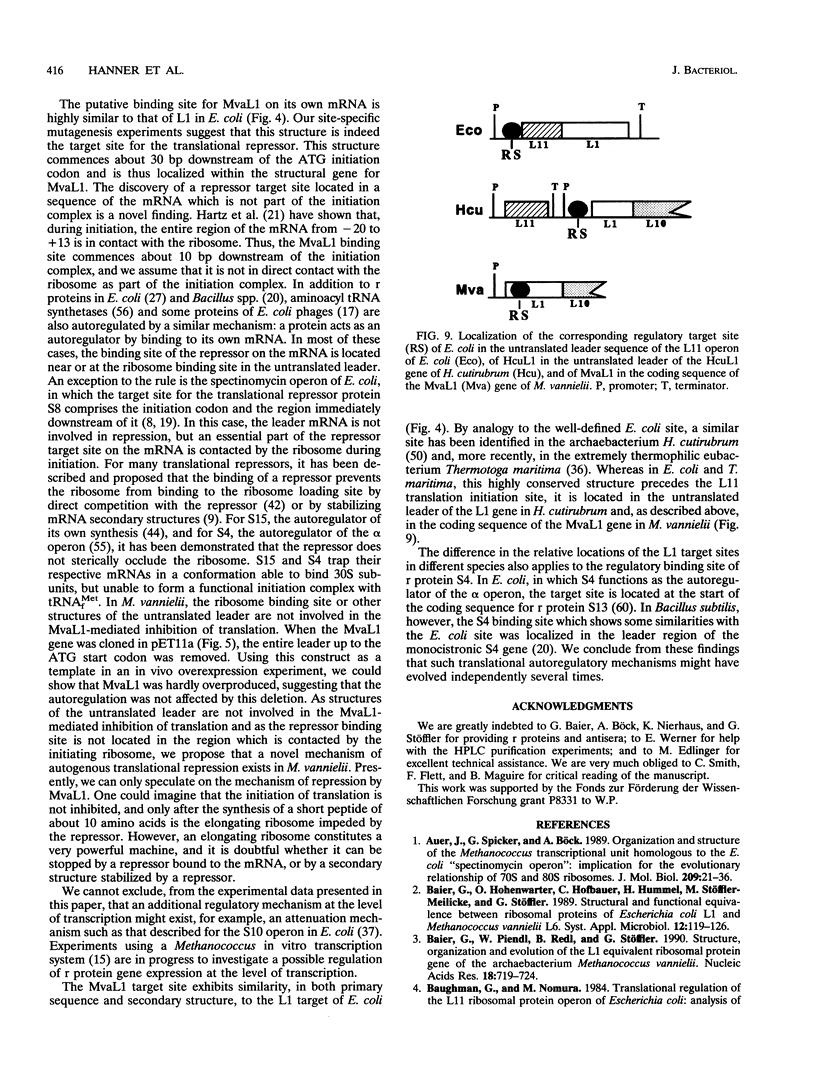
Abstract
The mechanisms for regulation of ribosomal gene expression have been characterized in eukaryotes and eubacteria, but not yet in archaebacteria. We have studied the regulation of the synthesis of ribosomal proteins MvaL1, MvaL10, and MvaL12, encoded by the MvaL1 operon of Methanococcus vannielii, a methanogenic archaebacterium. MvaL1, the homolog of the regulatory protein L1 encoded by the L11 operon of Escherichia coli, was shown to be an autoregulator of the MvaL1 operon. As in E. coli, regulation takes place at the level of translation. The target site for repression by MvaL1 was localized by site-directed mutagenesis to a region within the coding sequence of the MvaL1 gene commencing about 30 bases downstream of the ATG initiation codon. The MvaL1 binding site on the mRNA exhibits similarity in both primary sequence and secondary structure to the L1 regulatory target site of E. coli and to the putative binding site for MvaL1 on the 23S rRNA. In contrast to other regulatory systems, the putative MvaL1 binding site is located in a sequence of the mRNA which is not in direct contact with the ribosome as part of the initiation complex. Furthermore, the untranslated leader sequence is not involved in the regulation. Therefore, we suggest that a novel mechanism of translational feedback regulation exists in M. vannielii.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
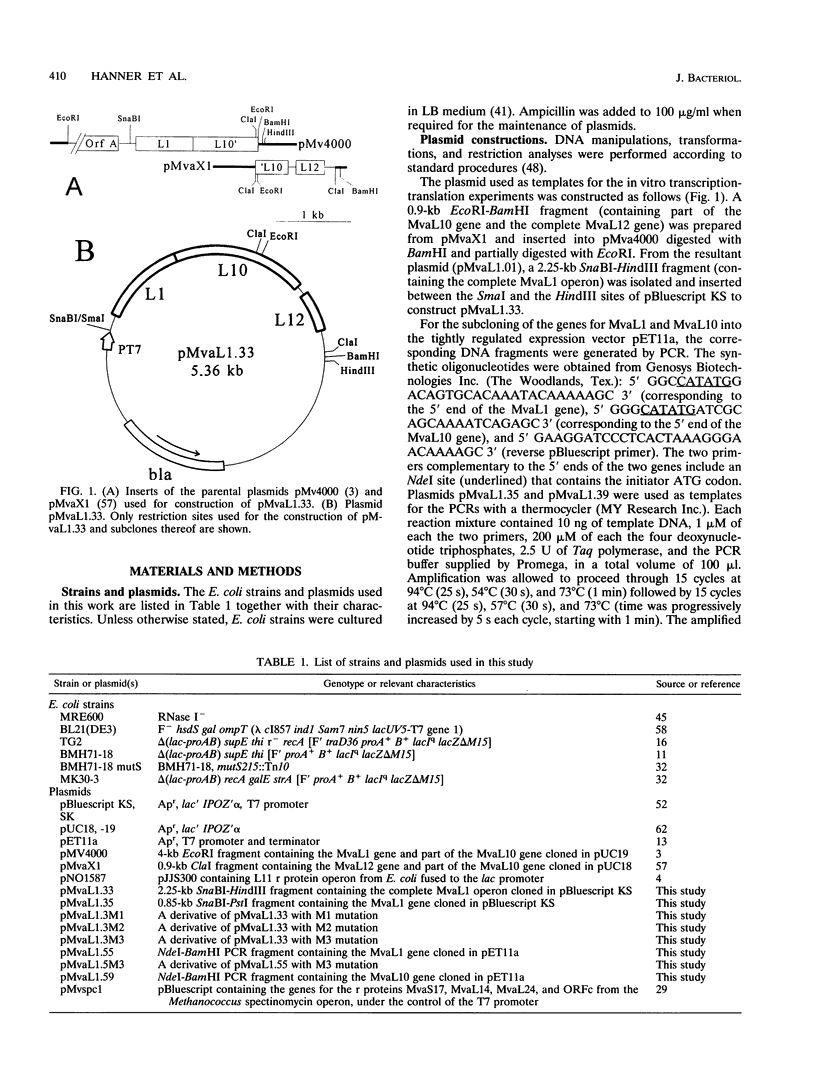
- Auer J., Spicker G., Böck A. Organization and structure of the Methanococcus transcriptional unit homologous to the Escherichia coli "spectinomycin operon". Implications for the evolutionary relationship of 70 S and 80 S ribosomes. J Mol Biol. 1989 Sep 5;209(1):21–36. doi: 10.1016/0022-2836(89)90167-8. [DOI] [PubMed] [Google Scholar]
- Baier G., Piendl W., Redl B., Stöffler G. Structure, organization and evolution of the L1 equivalent ribosomal protein gene of the archaebacterium Methanococcus vannielii. Nucleic Acids Res. 1990 Feb 25;18(4):719–724. doi: 10.1093/nar/18.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Translational regulation of the L11 ribosomal protein operon of Escherichia coli: analysis of the mRNA target site using oligonucleotide-directed mutagenesis. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5389–5393. doi: 10.1073/pnas.81.17.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Autogenous control of Escherichia coli ribosomal protein L10 synthesis in vitro. Proc Natl Acad Sci U S A. 1980 May;77(5):2592–2595. doi: 10.1073/pnas.77.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiano C., Matheson A. T., Traut R. R. Occurrence in the archaebacterium Sulfolobus solfataricus of a ribosomal protein complex corresponding to Escherichia coli (L7/L12)4.L10 and eukaryotic (P1)2/(P2)2.P0. J Biol Chem. 1990 Nov 5;265(31):18757–18761. [PubMed] [Google Scholar]
- Cerretti D. P., Mattheakis L. C., Kearney K. R., Vu L., Nomura M. Translational regulation of the spc operon in Escherichia coli. Identification and structural analysis of the target site for S8 repressor protein. J Mol Biol. 1988 Nov 20;204(2):309–329. doi: 10.1016/0022-2836(88)90578-5. [DOI] [PubMed] [Google Scholar]
- Christensen T., Johnsen M., Fiil N. P., Friesen J. D. RNA secondary structure and translation inhibition: analysis of mutants in the rplJ leader. EMBO J. 1984 Jul;3(7):1609–1612. doi: 10.1002/j.1460-2075.1984.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Molecular biology of archaebacteria. J Bacteriol. 1986 Nov;168(2):471–478. doi: 10.1128/jb.168.2.471-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing W. L., Dennis P. P. Transcription products from the rplKAJL-rpoBC gene cluster. J Mol Biol. 1987 Apr 20;194(4):609–620. doi: 10.1016/0022-2836(87)90238-5. [DOI] [PubMed] [Google Scholar]
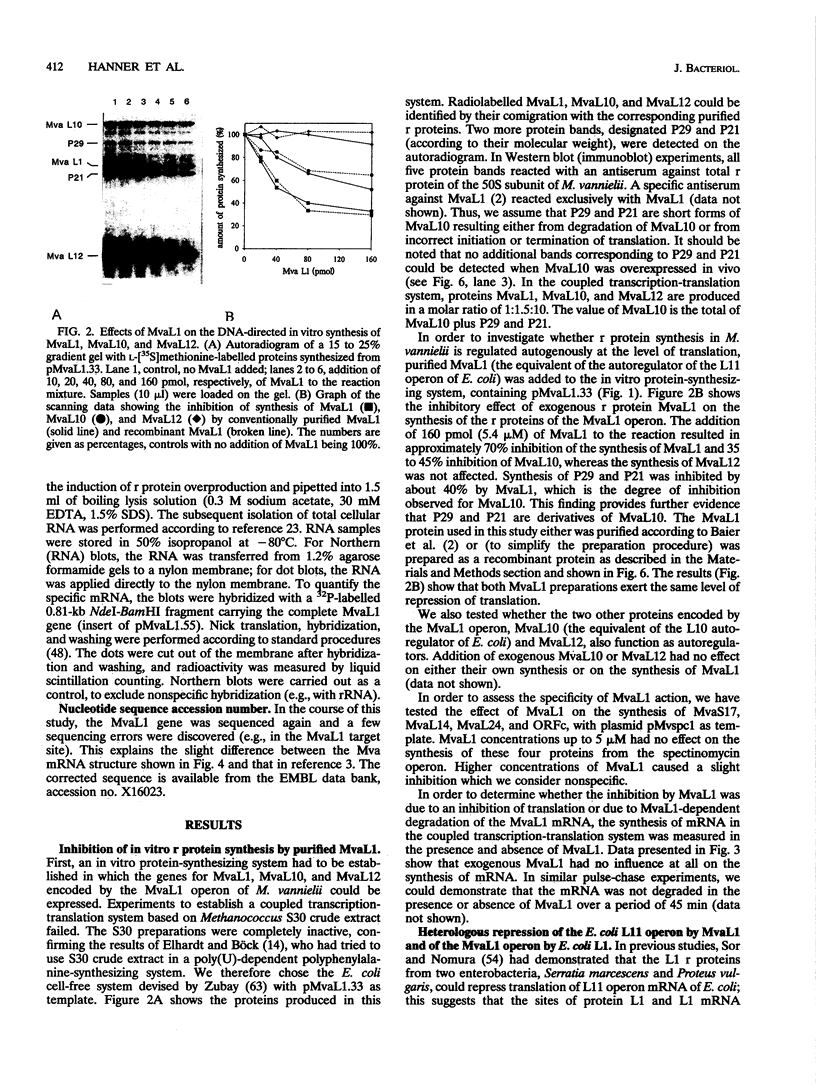
- Dubendorff J. W., Studier F. W. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol. 1991 May 5;219(1):45–59. doi: 10.1016/0022-2836(91)90856-2. [DOI] [PubMed] [Google Scholar]
- Frey G., Thomm M., Brüdigam B., Gohl H. P., Hausner W. An archaebacterial cell-free transcription system. The expression of tRNA genes from Methanococcus vannielii is mediated by a transcription factor. Nucleic Acids Res. 1990 Mar 25;18(6):1361–1367. doi: 10.1093/nar/18.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Cahill P. B., Thurlow D. L., Zimmermann R. A. Interaction of Escherichia coli ribosomal protein S8 with its binding sites in ribosomal RNA and messenger RNA. J Mol Biol. 1988 Nov 20;204(2):295–307. doi: 10.1016/0022-2836(88)90577-3. [DOI] [PubMed] [Google Scholar]
- Grundy F. J., Henkin T. M. The rpsD gene, encoding ribosomal protein S4, is autogenously regulated in Bacillus subtilis. J Bacteriol. 1991 Aug;173(15):4595–4602. doi: 10.1128/jb.173.15.4595-4602.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Hausner W., Frey G., Thomm M. Control regions of an archaeal gene. A TATA box and an initiator element promote cell-free transcription of the tRNA(Val) gene of Methanococcus vannielii. J Mol Biol. 1991 Dec 5;222(3):495–508. doi: 10.1016/0022-2836(91)90492-o. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Fukuda R. Autogenous and post-transcriptional regulation of RNA polymerase synthesis. Mol Cell Biochem. 1980 Aug 16;31(3):177–196. doi: 10.1007/BF00225850. [DOI] [PubMed] [Google Scholar]
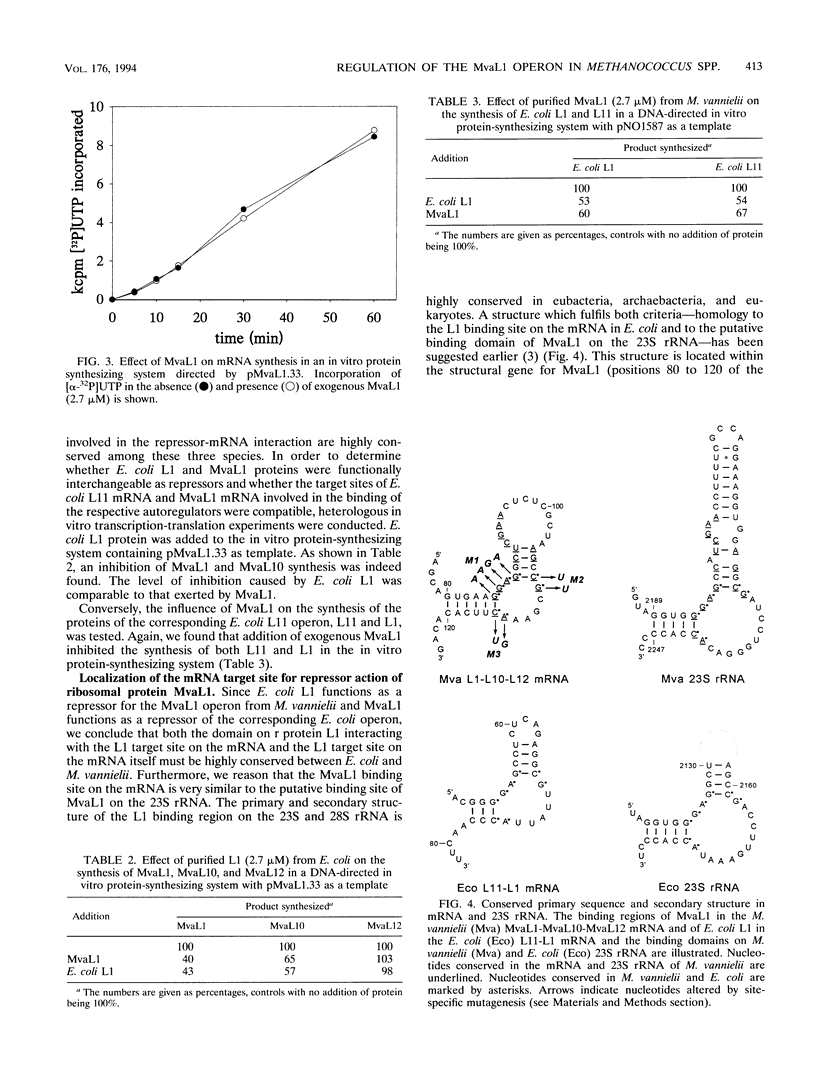
- Itoh T. Complete nucleotide sequence of the ribosomal 'A' protein operon from the archaebacterium, Halobacterium halobium. Eur J Biochem. 1988 Sep 15;176(2):297–303. doi: 10.1111/j.1432-1033.1988.tb14281.x. [DOI] [PubMed] [Google Scholar]
- Kearney K. R., Nomura M. Secondary structure of the autoregulatory mRNA binding site of ribosomal protein L1. Mol Gen Genet. 1987 Nov;210(1):60–68. doi: 10.1007/BF00337759. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpke A. K., Baier G., Wittmann-Liebold B. An archaebacterial gene from Methanococcus vannielii encoding a protein homologous to the ribosomal protein L10 family. FEBS Lett. 1989 Apr 24;247(2):167–172. doi: 10.1016/0014-5793(89)81326-2. [DOI] [PubMed] [Google Scholar]
- Köpke A. K., Paulke C., Gewitz H. S. Overexpression of the methanococcal ribosomal protein L12 in Escherichia coli and its incorporation into halobacterial 50 S subunits yielding active ribosomes. J Biol Chem. 1990 Apr 15;265(11):6436–6440. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechner K., Heller G., Böck A. Organization and nucleotide sequence of a transcriptional unit of Methanococcus vannielii comprising genes for protein synthesis elongation factors and ribosomal proteins. J Mol Evol. 1989 Jul;29(1):20–27. doi: 10.1007/BF02106178. [DOI] [PubMed] [Google Scholar]
- Liao D., Dennis P. P. The organization and expression of essential transcription translation component genes in the extremely thermophilic eubacterium Thermotoga maritima. J Biol Chem. 1992 Nov 15;267(32):22787–22797. [PubMed] [Google Scholar]
- Lindahl L., Archer R., Zengel J. M. Transcription of the S10 ribosomal protein operon is regulated by an attenuator in the leader. Cell. 1983 May;33(1):241–248. doi: 10.1016/0092-8674(83)90353-7. [DOI] [PubMed] [Google Scholar]
- Matheson A. T. Structure, function and evolution of the archaeal ribosome. Biochem Soc Symp. 1992;58:89–98. [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann B., Ehresmann C. Escherichia coli threonyl-tRNA synthetase and tRNA(Thr) modulate the binding of the ribosome to the translational initiation site of the thrS mRNA. J Mol Biol. 1990 Nov 20;216(2):299–310. doi: 10.1016/S0022-2836(05)80321-3. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Philippe C., Eyermann F., Bénard L., Portier C., Ehresmann B., Ehresmann C. Ribosomal protein S15 from Escherichia coli modulates its own translation by trapping the ribosome on the mRNA initiation loading site. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4394–4398. doi: 10.1073/pnas.90.10.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V., Gerchman S. E. Cloning, sequencing, and overexpression of genes for ribosomal proteins from Bacillus stearothermophilus. J Biol Chem. 1991 Jan 15;266(2):880–885. [PubMed] [Google Scholar]
- Reeve J. N. Molecular biology of methanogens. Annu Rev Microbiol. 1992;46:165–191. doi: 10.1146/annurev.mi.46.100192.001121. [DOI] [PubMed] [Google Scholar]
- Shimmin L. C., Dennis P. P. Characterization of the L11, L1, L10 and L12 equivalent ribosomal protein gene cluster of the halophilic archaebacterium Halobacterium cutirubrum. EMBO J. 1989 Apr;8(4):1225–1235. doi: 10.1002/j.1460-2075.1989.tb03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmin L. C., Newton C. H., Ramirez C., Yee J., Downing W. L., Louie A., Matheson A. T., Dennis P. P. Organization of genes encoding the L11, L1, L10, and L12 equivalent ribosomal proteins in eubacteria, archaebacteria, and eucaryotes. Can J Microbiol. 1989 Jan;35(1):164–170. doi: 10.1139/m89-025. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sor F., Nomura M. Cloning and DNA sequence determination of the L11 ribosomal protein operon of Serratia marcescens and Proteus vulgaris: translational feedback regulation of the Escherichia coli L11 operon by heterologous L1 proteins. Mol Gen Genet. 1987 Nov;210(1):52–59. doi: 10.1007/BF00337758. [DOI] [PubMed] [Google Scholar]
- Spedding G., Gluick T. C., Draper D. E. Ribosome initiation complex formation with the pseudoknotted alpha operon messenger RNA. J Mol Biol. 1993 Feb 5;229(3):609–622. doi: 10.1006/jmbi.1993.1067. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Butler J. S., Grunberg-Manago M. Genetic definition of the translational operator of the threonine-tRNA ligase gene in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4384–4388. doi: 10.1073/pnas.83.12.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel O., Köpke A. K., Kamp R. M., Böck A., Wittmann-Liebold B. Primary structure of the archaebacterial Methanococcus vannielii ribosomal protein L12. Amino acid sequence determination, oligonucleotide hybridization, and sequencing of the gene. J Biol Chem. 1988 May 15;263(14):6538–6546. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tang C. K., Draper D. E. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell. 1989 May 19;57(4):531–536. doi: 10.1016/0092-8674(89)90123-2. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Nomura M. Translational regulation of the L11 ribosomal protein operon of Escherichia coli: mutations that define the target site for repression by L1. Nucleic Acids Res. 1987 Apr 10;15(7):3085–3096. doi: 10.1093/nar/15.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]