Abstract
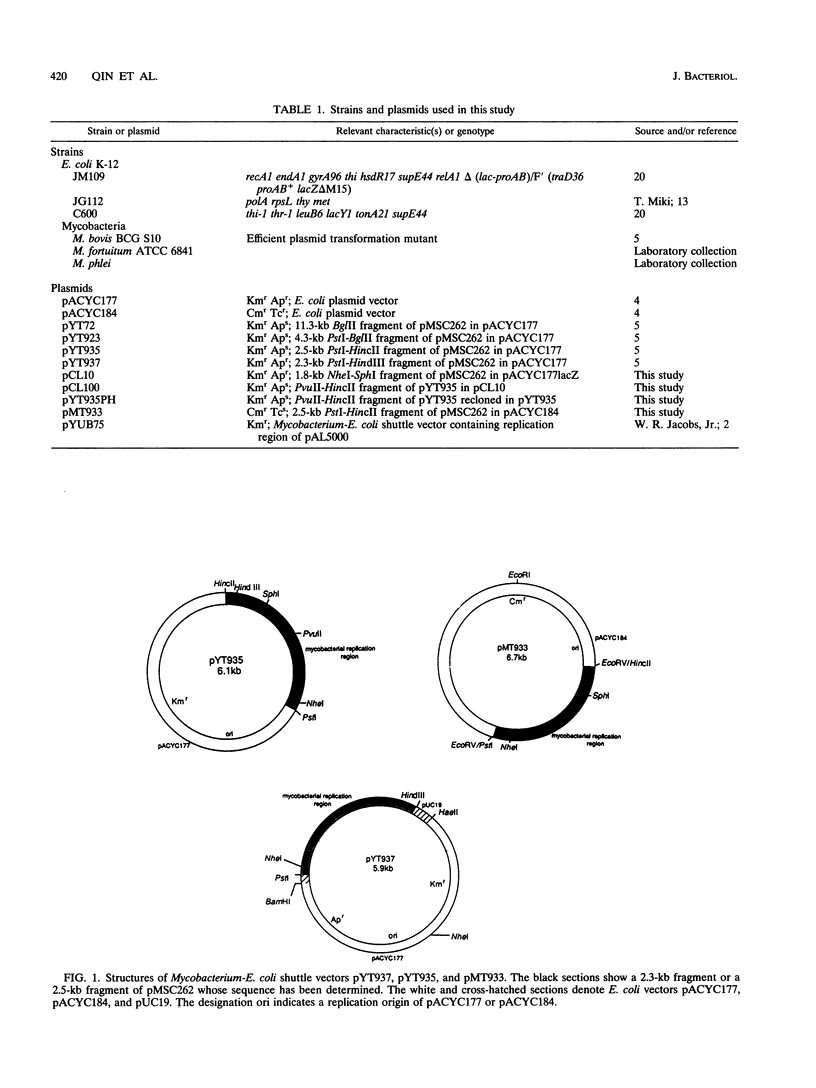
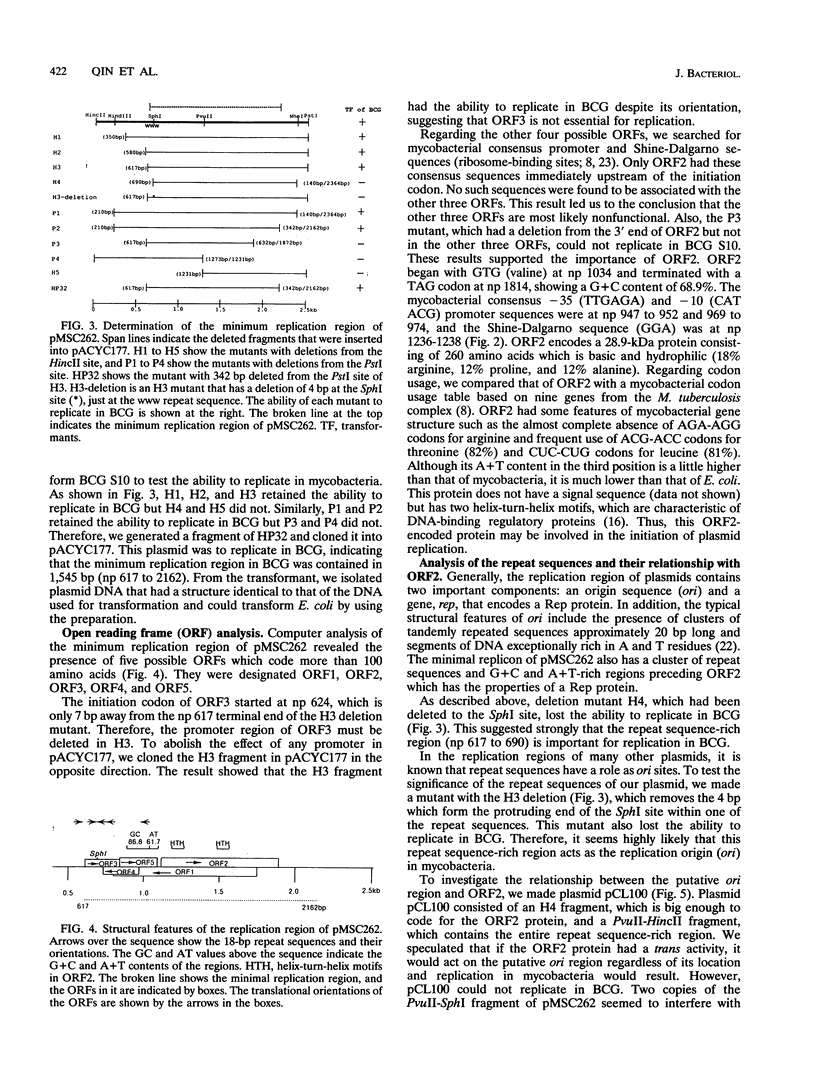
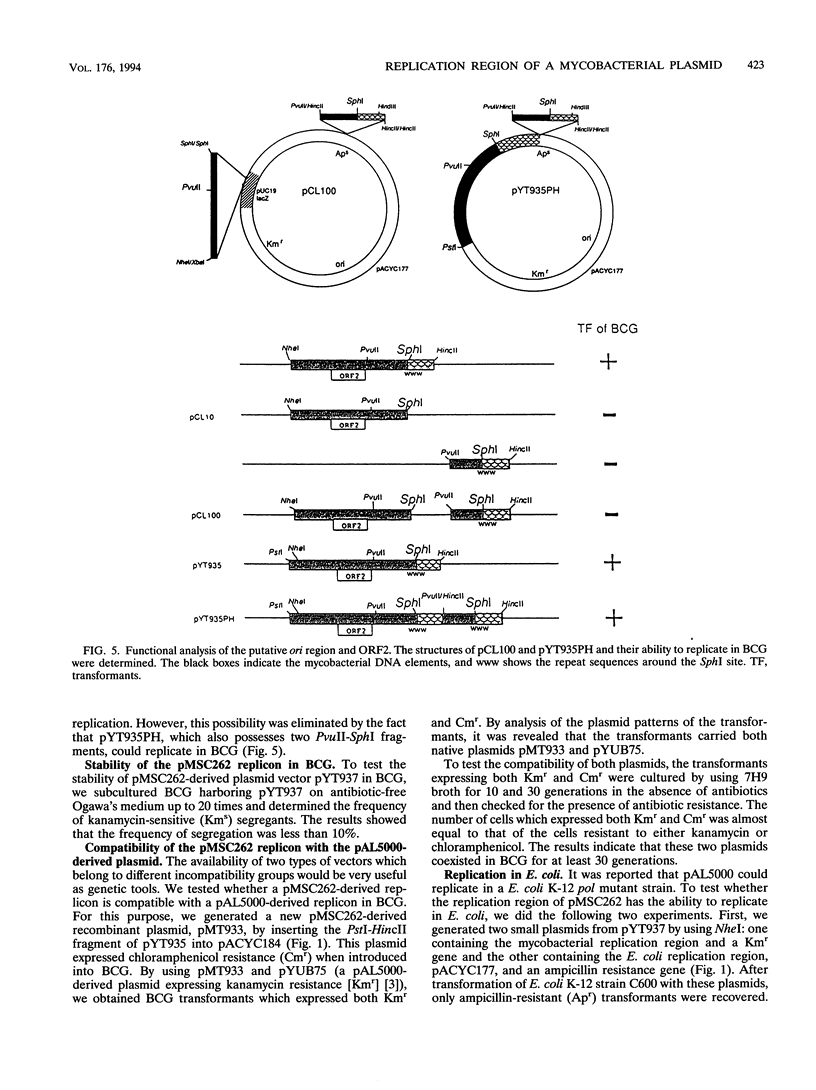
We determined the nucleotide sequence of a DNA fragment which contains the replication region of pMSC262, a Mycobacterium scrofulaceum plasmid used to construct the Mycobacterium-Escherichia coli shuttle vector. The complete sequence of the fragment contained 2,504 bp with an overall G+C content of 69.8%. By deletion analysis, we found that the minimum length required for plasmid replication in M. bovis BCG was about 1.6 kb. Within this region, several open reading frames (ORFs) and a putative replication origin (ori) were identified by computer analysis. One of the ORFs, ORF2, which encodes a putative 28.9-kDa basic protein with characteristics of DNA-binding proteins, appeared to be involved in replication of the plasmid in BCG. By separation of ORF2 and the putative ori region, it was revealed that the relative locations of ORF2 and the putative ori region are likely important for replication in BCG. No DNA or amino acid homologies were found between this replication region and that of pAL5000, another mycobacterial plasmid used for vector plasmid construction. In addition, we found that this replicon did not lead to replication in E. coli and was compatible in BCG with pAL5000-derived vector plasmid pYUB75 (R. G. Barletta, D. D. Kim, S. B. Snapper, B. R. Bloom, and W. R. Jacobs, J., J. Gen. Microbiol. 138:23-30, 1992).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I. Deoxyribonucleic acid relatedness among species of rapidly growing mycobacteria. Acta Pathol Microbiol Immunol Scand B. 1982 Oct;90(5):371–375. doi: 10.1111/j.1699-0463.1982.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Barletta R. G., Kim D. D., Snapper S. B., Bloom B. R., Jacobs W. R., Jr Identification of expression signals of the mycobacteriophages Bxb1, L1 and TM4 using the Escherichia-Mycobacterium shuttle plasmids pYUB75 and pYUB76 designed to create translational fusions to the lacZ gene. J Gen Microbiol. 1992 Jan;138(1):23–30. doi: 10.1099/00221287-138-1-23. [DOI] [PubMed] [Google Scholar]
- Baulard A., Jourdan C., Mercenier A., Locht C. Rapid mycobacterial plasmid analysis by electroduction between Mycobacterium spp. and Escherichia coli. Nucleic Acids Res. 1992 Aug 11;20(15):4105–4105. doi: 10.1093/nar/20.15.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans J., Martin C., Huijberts G. N., Goosen T., de Bont J. A. Transformation of Mycobacterium aurum and Mycobacterium smegmatis with the broad host-range gram-negative cosmid vector pJRD215. Mol Microbiol. 1991 Jun;5(6):1561–1566. doi: 10.1111/j.1365-2958.1991.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Hinshelwood S., Stoker N. G. An Escherichia coli-Mycobacterium shuttle cosmid vector, pMSC1. Gene. 1992 Jan 2;110(1):115–118. doi: 10.1016/0378-1119(92)90453-v. [DOI] [PubMed] [Google Scholar]
- Kües U., Stahl U. Replication of plasmids in gram-negative bacteria. Microbiol Rev. 1989 Dec;53(4):491–516. doi: 10.1128/mr.53.4.491-516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labidi A., Mardis E., Roe B. A., Wallace R. J., Jr Cloning and DNA sequence of the Mycobacterium fortuitum var fortuitum plasmid pAL5000. Plasmid. 1992 Mar;27(2):130–140. doi: 10.1016/0147-619x(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Lazraq R., Clavel-Sérès S., David H. L., Roulland-Dussoix D. Conjugative transfer of a shuttle plasmid from Escherichia coli to Mycobacterium smegmatis [corrected]. FEMS Microbiol Lett. 1990 May;57(1-2):135–138. doi: 10.1016/0378-1097(90)90427-r. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Pascopella L., Jacobs W. R., Jr, Hatfull G. F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guérin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Manis J., Kline B., Bishop A. Nonintegrated plasmid-folded chromosome complexes: genetic studies on formation and possible relationship to plasmid replication. Plasmid. 1978 Jun;1(3):273–283. doi: 10.1016/0147-619x(78)90045-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Radford A. J., Hodgson A. L. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid. 1991 Mar;25(2):149–153. doi: 10.1016/0147-619x(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Ranes M. G., Rauzier J., Lagranderie M., Gheorghiu M., Gicquel B. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a "mini" mycobacterium-Escherichia coli shuttle vector. J Bacteriol. 1990 May;172(5):2793–2797. doi: 10.1128/jb.172.5.2793-2797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzier J., Moniz-Pereira J., Gicquel-Sanzey B. Complete nucleotide sequence of pAL5000, a plasmid from Mycobacterium fortuitum. Gene. 1988 Nov 30;71(2):315–321. doi: 10.1016/0378-1119(88)90048-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Regulation of plasmid replication. Microbiol Rev. 1984 Mar;48(1):1–23. doi: 10.1016/b978-0-12-048850-6.50006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Villar C. A., Benitez J. Functional analysis of pAL5000 plasmid in Mycobacterium fortuitum. Plasmid. 1992 Sep;28(2):166–169. doi: 10.1016/0147-619x(92)90047-e. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zainuddin Z. F., Kunze Z. M., Dale J. W. Transformation of Mycobacterium smegmatis with Escherichia coli plasmids carrying a selectable resistance marker. Mol Microbiol. 1989 Jan;3(1):29–34. doi: 10.1111/j.1365-2958.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]