Abstract
Although many types of membrane-bound organelles rely upon microtubule-based transport for their proper placement within the cytoplasm, the molecular mechanisms that regulate intracellular motility remain largely unknown. To address this problem, we have studied the microtubule-dependent dispersion and aggregation of pigment granules from an immortalized Xenopus melanophore cell line. We have reconstituted pigment granule motility along bovine brain microtubules in vitro using a microscope-based motility assay. Pigment granules, or melanosomes, move along single microtubules bidirectionally; however, analysis of the polarities of this movement shows that melanosomes that have been purified from dispersed cells exhibit mostly plus end-directed motility, while movement of organelles from aggregating cells is biased toward the minus end. Removal of all soluble proteins from the melanosome fractions by density gradient centrifugation does not diminish organelle motility, demonstrating that all the components required for transport have a stable association with the melanosome membranes. Western blotting shows the presence of the plus end-directed motor, kinesin-II, and the minus end-directed motor, cytoplasmic dynein in highly purified melanosomes. Therefore, purified melanosomes retain their ability to move along microtubules as well as their regulated state. Direct biochemical comparison of melanosomes from aggregated and dispersed cells may elucidate the molecular mechanisms that regulate organelle transport in melanophores.
Microtubule-based motor proteins have been implicated in the bidirectional transport and localization of many organelles within the eukaryotic cytoplasm. During interphase, microtubule motor proteins are thought to determine the distributions and structures of membranous organelles such as the endoplasmic reticulum, the Golgi apparatus, and lysosomes (1, 2). Dynamic processes such as fast axonal transport, endocytosis, secretion, and intercompartmental trafficking are also mediated by motor proteins (3, 4). During cell division, motors participate in the assembly of the spindle and have been implicated in chromosome congression to the metaphase plate as well as poleward transport during anaphase (5). The complexity of these processes suggests that motor-mediated transport is subject to precise spatial and temporal regulation. Despite this fact, the regulatory mechanisms that govern intracellular motility remain poorly understood.
Melanophores offer a promising model system to address the issue of motor regulation (6). The sole physiological role of these cells is the simultaneous transport of hundreds of membrane-bound pigmented organelles, termed melanosomes, either to amass at the center of the cell or to disperse throughout the cytoplasm. The net effect of this transport is to give lower vertebrates, such as fish and amphibians, the ability to change color. The animals appear darker when the cells have dispersed pigment and lighter when the cells have aggregated pigment. Melanophores transport melanosomes along a highly developed radially organized microtubule cytoskeleton. As with most cell types, the microtubules are oriented with their minus ends associated with a perinuclear microtubule-organizing center and their plus ends extending out to the cell periphery (7). Melanophores regulate the direction of pigment transport by modulating the intracellular second messenger, cAMP (8, 9). Upon stimulation of the cells with the appropriate hormonal stimulus, adenylate cyclase activity is up-regulated, thereby increasing cytosolic cAMP levels. Increased cAMP activates pigment dispersion, most likely through the activation of cAMP-dependent protein kinase (PKA; refs. 9 and 10). Conversely, a decrease of cAMP levels within the cell permits an as yet unidentified phosphatase to trigger pigment aggregation (11, 12). It is unknown whether the motors responsible for pigment transport are phosphorylated directly by PKA or are downstream in a multistep pathway.
The development of an immortalized melanophore cell line derived from Xenopus melanophores by Lerner and coworkers (10) has made it possible to produce homogeneous cultures in quantities that facilitate molecular approaches. We have used this melanophore cell line as a model to study the mechanisms regulating organelle transport. Here we report that melanosome-associated motors retain their regulated states and recapitulate the polarity of their transport when examined in an in vitro motility assay. Comparison of the motors present on melanosomes from dispersed and aggregated cells should elucidate the molecular mechanisms that govern the directionality of pigment granule transport.
MATERIALS AND METHODS
Materials.
Taxol was the gift of N. Lomax at the National Cancer Institute. Bovine brain tubulin was purified by polymerization–depolymerization cycles and phosphocellulose chromatography and stored in liquid nitrogen (13). Axonemes were purified from sea urchin sperm (a gift from Dan Buster and Jon Scholey, Division of Molecular and Cellular Biology, University of California, Davis; ref. 14). All other reagents were purchased from Sigma unless otherwise noted.
Cell Culture.
Immortalized Xenopus melanophores (gift of Michael Lerner, Yale University School of Medicine) were cultured at 27°C in 0.7 × L-15 medium (GIBCO/BRL), supplemented with 10% fetal bovine serum, 5 μg/ml insulin, penicillin, and streptomycin as described (10). Cells for experiments were routinely grown to near confluence in 10-cm tissue culture dishes. Melanophores were induced to aggregate or disperse their pigment by replacement of the growth medium with serum-free 0.7 × L-15 medium containing 10 nM melatonin or 1 mM 3-isobutyl-1-methyl xanthine (IBMX), respectively.
Isolation of Melanosomes.
The melanosome purification relied upon the separation of pigment granules from other cellular components by Percoll density gradient centrifugation. Melanosomes were isolated using one of two buffers depending on the experimental treatment. For immunoblotting, buffer B (50 mM Hepes, pH 7.5/50 mM KCl/1 mM MgCl2) was used, while for motility assays, IMB50 (50 mM imidazole, pH 7.4/1 mM EGTA/0.5 mM EDTA/5 mM magnesium acetate/175 mM sucrose/150 μg/ml casein/1 mM 2-mercaptoethanol) was used. Confluent melanophores were rinsed with buffer and scraped into 3 ml of buffer supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride and 10 μg/ml each pepstatin, leupeptin, and chymostatin). The cells were lysed by 5–10 passes through a 25-gauge hypodermic needle. The cell lysate was further diluted into 10 ml of buffer and centrifuged at 750 × g for 5 min at 4°C in a JS 13.1 rotor (Beckman) to pellet cell debris and nuclei. Melanosomes were collected from the supernatant by pelleting at 2,500 × g for 5 min in the same rotor. The supernatant from the second spin was removed and the melanosome pellet was gently resuspended. This crude melanosome fraction was further purified by centrifugation at 2,500 × g for 15 min through a cushion of 80% Percoll in buffer.
For assays comparing the motility of pigment granules from aggregated and dispersed melanophores, the cells were pretreated for 90 min with 10 nM melatonin or 1 mM IBMX in serum-free medium, respectively. Melanosomes were purified in IMB50 supplemented with 10 nM melatonin for aggregated cells or 1 mM IBMX, 0.5 mM ATP, and 0.5 mM cAMP for dispersed cells.
Purified melanosome pellets were prepared for transmission electron microscopy by fixation with 2% glutaraldehyde in buffer B for 2 h at 4°C, followed by 1% osmium tetroxide for 20 min. The fixed pellets were dehydrated in ethanol and propylene oxide, embedded in Medcast resin, thin-sectioned, and examined using a Hitachi H-600 electron microscope at an accelerating voltage of 100 kV.
For analysis by SDS/PAGE and immunoblotting, melanosome pellets were resuspended in SDS/sample buffer and heated at 100°C for 5 min.
Reconstituted Motility Assay.
A diamond scribe was used to etch circles 12 mm in diameter in the center of standard microscope slides (Corning), and the slides were cleaned overnight in chromic acid. On the day of the experiments, they were rinsed extensively with deionized water, dried in an oven, and made adhesive for microtubules or axonemes in one of two ways: (i) the slides were placed in a humidified chamber and a drop of 5 mg/ml DEAE-dextran (Pharmacia) in water was applied to the etched circles. After 10 min, the drops were aspirated and the slides were briefly rinsed with deionized water and air-dried; or (ii) the slides were placed in an airtight box and a drop of α-aminopropyltriethoxysilane was applied to the etched circle for 5 min (15). Following an extensive rinse with ethanol, the slides were cured at 100°C for 1 h to crosslink the silane to the glass. Modified slides were stored in a dust-free, airtight box until use.
Motility assays were performed within 5-μl flow chambers constructed from treated slides and 22-cm2 no. 1 coverslips (Corning) separated by two parallel strips of Apiezon M grease (M & I Materials, Manchester, England). DEAE-dextran slides were used for assays to determine the total activity of melanosome fractions. The chambers were filled with BRB80 (80 mM Pipes, pH 6.9/1 mM EGTA/1 mM MgCl2) supplemented with 10 μM taxol and imaged by video-enhanced differential–interference contrast (DIC) microscopy. Taxol-stabilized microtubules were prepared from phosphocellulose-purified bovine brain tubulin to a final concentration of 2 mg/ml. Five microliters of the microtubule suspension was perfused into the chamber to attach to the dextran-coated slide to form a continuous carpet of microtubules oriented parallel to the direction of flow. Unbound microtubules were removed and the exposed charged surface of the glass was blocked by perfusion with four volumes of 15 mg/ml casein in IMB50 and 5 μM taxol. For assays to examine the polarity of melanosome transport, chambers were constructed using aminosilanized slides. The chambers were first filled with BRB80 plus 10 μM taxol, as above, and imaged on the microscope. Axoneme-nucleated microtubules were prepared using N-ethyl maleimide-treated tubulin as described (16) and perfused into the chamber until they attached at a density of about one axoneme per field. Unbound axonemes and free microtubules were washed and the glass was blocked as above.
Melanosomes were typically diluted to an OD550 of ≈0.16 into 20 μl of IMB50 containing 2 mM ATP. Five-microliter aliquots of this dilute melanosome suspension were perfused into the chambers for each assay. To keep the organelles in suspension, fresh perfusions of 5 μl were made every 5 min.
Video Microscopy.
In vitro melanosome motility was observed by video-enhanced DIC microscopy using a Nikon Microphot-SA equipped with a Planapochromatic 60×/1.4 NA oil immersion objective lens, a 4× projection lens, DIC prisms, and a 1.4 NA oil-immersion condenser. A metal halide lamp (NMH-1) equipped with a liquid fiber optic scrambler was used for illumination (Nikon). Images were captured with a C2400 Newvicon camera, while contrast enhancement and frame averaging were performed using an Argus-10 video processor (Hamamatsu, Hamamatsu City, Japan). Motility was recorded onto super-VHS format tapes using a time-lapse S-VHS recorder (Panasonic).
PAGE and Immunoblotting.
Samples were analyzed by discontinuous SDS/PAGE on 5–15% gradient polyacrylamide gels (17). Immunoblotting was performed according to the protocol of Towbin et al. (18). Tyrosinase was detected using the polyclonal antibody PEP7, provided by Vincent Hearing (National Institutes of Health, Bethesda; ref. 19). Kinesin was probed with rabbit polyclonal HD-kin5 provided by Fatima Gyoeva (Institute of Protein Research, Russian Academy of Sciences, Moscow; ref. 20). Kinesin-related proteins were detected with the pan-kinesin antibodies HIPYR and LAGSE from Ken Sawin and Tim Mitchison (University of California, San Francisco; refs. 21 and 22). Kinesin-II was detected with the monoclonal K2.4 provided by Jonathan Scholey (University of California, Davis; ref. 23). Cytoplasmic dynein was probed with the monoclonal 74.1 against dynein intermediate chain from Kevin Pfister (University of Virginia Health Sciences Center, Charlottesville; ref. 24). Blots were probed with appropriate horseradish peroxidase-conjugated secondary antibodies and visualized using the SuperSignal CL-HRP chemiluminescent detection system (Pierce). To determine the amount of kinesin-II and dynein present on melanosomes, we used quantitative immunoblotting. The intensity of the bands was measured on a Macintosh computer using the public domain National Institutes of Health image program. The yield of purified melanosomes was measured by comparing the melanin absorbance at 550 nm in crude cell extracts and Percoll-purified organelles.
RESULTS
Pigment Granule Transport in Living Xenopus Melanophores.
Melanophores were observed by video-enhanced brightfield microscopy using low-intensity illumination and a far-red filter (RG 695; Chroma Technology, Brattleboro, VT) to eliminate the light-induced pigment dispersion described for Xenopus melanophores (10). The cells usually grow with their pigment uniformly dispersed throughout the cytoplasm but are induced to aggregate upon the addition of 10 nM melatonin. Melanosomes are transported toward the center of the cell in short movements punctuated by brief pauses, or saltations. Individual pigment granules are often seen to reverse direction, moving toward the cell periphery for ≈1–3 μm before resuming their centripetal movement. During aggregation, melanosomes are transported to the cell center at a velocity of 0.35 μm/s (SD, ±0.14), while the brief sprints toward the periphery of the cell have a velocity of 0.22 μm/s (SD, ±0.13). Pigment aggregation is complete in 30–45 min. Melanosomes usually collect into a perinuclear mass, although individual organelles sometimes separate from the pigment mass for brief excursions toward the periphery. If melatonin is washed out and replaced with medium supplemented with 1 mM IBMX, a phosphodiesterase inhibitor, melanophores disperse their pigment outwards to the periphery and into the cells’ many fine processes. Melanosome transport during dispersion is also saltatory with brief reversals of direction. Complete dispersion occurs in ≈45 min. Centrifugal transport exhibits a velocity of 0.13 μm/s (SD, ±0.07), with short movements toward the cell center occurring at a rate of 0.16 μm/s (SD, ±0.06). Depolymerization of microtubules with 10 μM nocodozole for 3 h inhibits both melanosome aggregation and dispersion, verifying that melanosome transport is microtubule-based (data not shown).
Purification of Melanosomes.
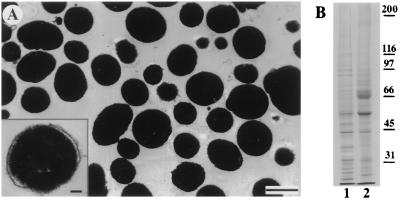
To pursue a molecular characterization of the motors involved with pigment granule transport, we developed protocols for the mass isolation and purification of melanosomes using density gradient centrifugation. Soluble proteins and vesicles are retained in the supernatant, while most of the melanosomes sediment through the cushion to collect at the bottom of the tube. Electron microscopic examination of the melanosome pellets shows that they are uncontaminated by vesicles or other organelles (Fig. 1A). Individual melanosomes have a spherical shape with a uniform diameter of ≈0.5 μm. Most of the organelles purified in this way possess intact membrane bilayers that completely envelop an osmiophillic core of melanin (Fig. 1A Inset). Melanosomes, therefore, possess a higher buoyant density than other cellular components and may be effectively purified in one step by using density gradient centrifugation.
Figure 1.
(A) Electron micrograph of a thin-sectioned pellet of purified melanosomes. The purified melanosome fractions are uncontaminated by other organelles. (Bar = 0.5 μm.) (Inset) A melanosome at higher magnification showing bilayer membrane encapsulating the electron-dense melanin core. (Bar = 0.1 μm.) (B) A Coomassie-stained polyacrylamide gel comparing melanophore extract (lane 1) with purified melanosomes (lane 2). The positions of the molecular weight markers are indicated to the right of lane 2.
The protein composition of purified melanosomes was analyzed on Coomassie-stained SDS/PAGE gels. Melanosomes have a less complex profile than whole cell extracts; certain abundant cytosolic proteins are absent from the pellet, while other protein bands become enriched (Fig. 1B). The most notable differences are an enrichment for major proteins of ≈55 and 70 kDa, as well as several minor species. The 70-kDa polypeptide is the most abundant protein in purified melanosome fractions and was identified as the glycoprotein tyrosinase by immunoblotting with a polyclonal antibody, PEP7 (19). This enzyme is present in association with the membranes of melanosomes, where it plays a key role in melanin synthesis and is, therefore, a good marker enzyme for melanosomes (25). Immunoblots probed with a monoclonal antibody against α-tubulin, confirmed that soluble proteins are excluded by the Percoll step (data not shown). The purified melanosome fractions obtained by density gradient centrifugation are, therefore, highly purified and morphologically intact.
Regulation of Melanosome Transport Along Microtubules In Vitro.
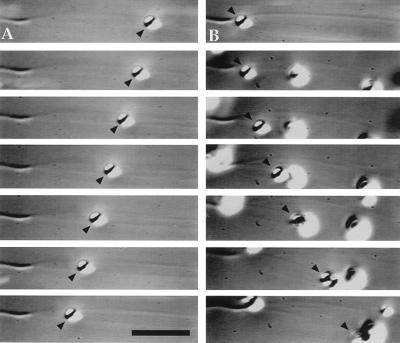
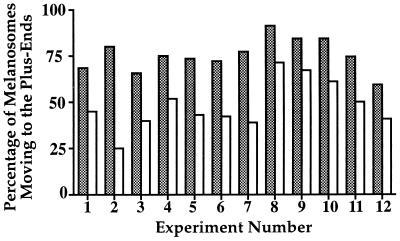
We have developed a motility assay to directly examine the activity and regulation of melanosome-associated motors in vitro. Crude melanosome fractions centrifuged from the cell lysate without density gradient purification are supplemented with 2 mM Mg-ATP and combined with axoneme-nucleated microtubules attached to the bottom of a microscope flow chamber. Perfused melanosomes drift in Brownian motion until encountering microtubules, at which point they attach and exhibit motility that is bidirectional with respect to microtubule polarity (Fig. 2). Pigment granules purified from melanophores that had either been induced to disperse with 1 mM IBMX or that aggregate with 10 nM melatonin were tested in parallel in the motility assay on axoneme-nucleated microtubules. In both cases, melanosomes exhibit bidirectional motility. However, there is a reproducible difference in the proportion of organelles moving in each direction between the organelles from aggregated and dispersed melanophores. Melanosomes purified from dispersed cells move predominantly toward the microtubule plus ends, while the motility of melanosomes obtained from aggregated cells is more biased toward the minus ends. Although the ratio of plus end- to minus end-directed motility varied between different experiments, in all trials performed, the ratio of plus end- to minus end-directed particles was always greater for organelles purified from the cells treated with IBMX than the cells treated with melatonin (Fig. 3). We conclude that populations of melanosomes purified from aggregated and dispersed cells are functionally different and that the differences we observe in the polarity of their motility reflects the regulated states of the motors in the cell.
Figure 2.
Bidirectional motility of a single melanosome along an axoneme-nucleated microtubule observed by video-enhanced DIC microscopy. Portions of a video sequence showing (A) minus end-directed motility and (B) plus end-directed motility of the same melanosome (arrowheads). The elapsed time between frames is 2 s. (Bar = 6 μm.)
Figure 3.
Comparison of the polarity of movement between melanosomes isolated from dispersed (shaded bars) and aggregated (open bars) melanophores. Results of twelve independent experiments are shown. Melanosome motility was assayed on axoneme-nucleated microtubules, and at least four assays per treatment were performed for each experiment. The polarity of each movement was scored and the percentage of plus end-directed motility was determined for each trial.
The movement of individual melanosomes is usually processive, continuing in a single direction over many microns. On rare occasions, some pigment granules are observed to pause and reverse directions on a single microtubule during the course of their movement. Plus end-directed melanosomes move with an average velocity of 0.65 μm/s (SD, ±0.21), while minus end-directed melanosomes travel at an average velocity of 1.15 μm/s (SD, ±0.43). Pigment granule motility is absolutely dependent upon nucleotide hydrolysis, as omission of ATP or substitution of 5′-adenylyl-β,γ-imidodiphosphate (AMP-PNP), a nonhydrolyzable analog of ATP, causes melanosomes to bind tightly to microtubules in a rigor-like state. When 2 mM GTP was substituted for ATP, melanosomes were observed to exhibit motility toward microtubule plus ends exclusively. Motility assays performed in the presence of 2 mM ATP and 30 μM sodium orthovanadate, an inhibitor of dynein, likewise exhibited only plus end-directed transport.
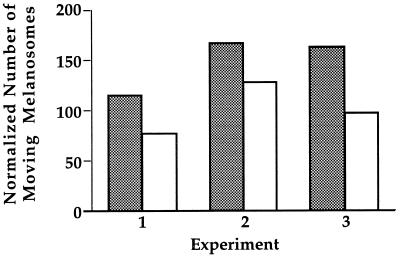
To test if melanosome-associated motors required soluble factors to promote motility, Percoll-purified melanosomes were tested using the motility assay. We found that, as in the case of the crude melanosomes, Percoll-purified melanosomes exhibit vigorous motility along microtubules toward both the plus and minus ends. To quantitatively compare the motility of the crude and Percoll-purified fractions, melanosome transport was assayed on continuous microtubule carpets. Due to their high buoyant density, melanosomes settle on the microtubules at the bottom of the chambers shortly after perfusion. The continuous layer of microtubules is a suitable substrate to quantify melanosome motility, as virtually every single organelle encounters microtubules. The total number of melanosomes that attach and exhibit motility can be counted and normalized to the melanosome concentration, as measured by their absorbance at 550 nm. The results of three independent experiments are presented in Fig. 4. These results show that Percoll purification does not significantly reduce overall motility of melanosomes, indicating that melanosome movement along microtubules does not require additional soluble proteins. The slight reduction of motility observed after Percoll purification can be attributed to the additional pelleting and resuspension of the fraction, as we have observed by electron microscopy that extensive shearing of melanosomes during pellet resuspension can strip membranes from their melanin cores (data not shown).
Figure 4.
Comparison of the movement of crude (shaded bars) and Percoll-purified melanosomes (open bars). The samples were assayed on a continuous carpet of microtubules, and the total number of motile melanosomes per field was tallied over 10-min periods. At least three different samples were assayed per preparation. The results for each treatment were averaged, and both results were normalized for melanosome concentration using the OD550 of each sample. Results of three individual experiments are shown.
To examine the effect of soluble melanophore proteins on motility, we conducted assays in the presence of cell extracts. Consistent with the results of our Percoll experiments, addition of soluble proteins did not affect the overall amount of melanosome motility as assayed on a microtubule carpet. Likewise, the ratio of plus end- to minus end- directed movement of organelles from aggregated or dispersed cells remained unaffected when tested on axoneme-nucleated microtubules.
Kinesin-II and Cytoplasmic Dynein Are Present on Melanosomes.
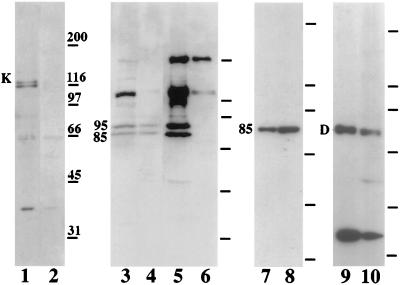
Immunoblots of Percoll-purified organelles were probed with antibodies that recognize known motor proteins. Conventional kinesin was detected with an affinity-purified polyclonal antibody, HD-kin5. This antibody recognizes a doublet of proteins with a molecular weight of ≈120 kDa in whole cell extracts (Fig. 5, lane 1) but, surprisingly, does not crossreact with purified melanosomes (Fig. 5, lane 2). In an attempt to identify other kinesin-like motors, blots were probed with HIPYR, a polyclonal antibody raised against a conserved peptide within the motor domains of kinesin-related proteins (21, 22). This antibody recognizes several proteins with molecular weights between 85 and 200 kDa in whole cell lysates (Fig. 5, lane 3) but reacts only with a doublet corresponding to proteins of 85 and 95 kDa in purified melanosome fractions (Fig. 5, lane 4). No other protein species in these fractions is recognized by HIPYR or by LAGSE, another pan-kinesin antibody (data not shown). To confirm the identities of the 85- and 95-kDa components as motor proteins, we tested their ability to bind to microtubules in the presence of the nonhydrolyzable ATP analog, AMP-PNP. Both of these proteins cosediment with taxol-stabilized microtubules in the presence of AMP-PNP (Fig. 5, lane 5) but not ATP (Fig. 5, lane 6). The molecular weights of these proteins suggested that they were the two motor subunits of the heterotrimeric motor kinesin-II (ref. 23; for review see ref. 26) This possibility was directly tested by immunoblotting with a monoclonal antibody, K2.4, which was raised against the 85-kDa subunit of sea urchin kinesin-II. This antibody clearly recognizes an 85-kDa component in melanosome preparations (Fig. 5, lane 8), which shows an enrichment over the cell extract (Fig. 5, lane 7), confirming that kinesin-II is indeed present on melanosomes. The 95-kDa protein recognized by HIPYR is, therefore, most likely the 95-kDa subunit of melanophore kinesin-II. Addition of the K2.4 monoclonal antibody to melanosomes did not inhibit plus end-directed motility in vitro, however.
Figure 5.
Microtubule motors in melanophores and on melanosomes. Lanes 1–10 show immunoblots probed with antibodies against motor proteins. Kinesin was probed with polyclonal HD-kin5 in cell extract (lane 1) and melanosomes (lane 2). Kinesin heavy chain is marked with “K.” Kinesin-related proteins were detected with the pan-kinesin antibody HIPYR in cell extract (lane 3), melanosomes (lane 4), and proteins that cosediment with microtubules in the presence of AMP-PNP (lane 5) or ATP (lane 6). The 85- and 95-kDa subunits of kinesin-II are indicated. Kinesin-II was probed with the monoclonal K2.4 in cell extract (lane 7) and melanosomes (lane 8). The position of the 85-kDa chain is indicated. Cytoplasmic dynein was detected with the monoclonal 74.1 against dynein intermediate chain in cell extract (lane 9) and melanosomes (lane 10). The position of the 83-kDa intermediate chain is indicated with “D.” The positions of the molecular weight standards are marked to the right of each blot.
The presence of the minus end-directed motor, cytoplasmic dynein, was tested by immunoblotting with the monoclonal antibody 74.1 (24). This antibody crossreacts with an intermediate chain of cytoplasmic dynein, which has an apparent molecular weight of 83 kDa in Xenopus (27). In melanophores, 74.1 also crossreacts with a low-molecular weight band of unknown identity (Fig. 5, lane 9). Both dynein intermediate chain and the low-molecular weight component copurify with melanosomes (Fig. 5, lane 10).
Using quantitative immunoblotting, we estimated the fraction of kinesin-II and dynein that copurifies with melanosomes. We found that typically ≈40% of kinesin-II and 40% of cytoplasmic dynein present in whole cell lysates is recovered in the purified melanosome fraction.
DISCUSSION
Melanosome-Associated Motor Activities.
We have established an assay to study the microtubule-based transport of pigment granules purified from Xenopus melanophores. Our results demonstrate the presence of two opposing motor activities on these organelles that may be differentially regulated. Immunoblots of purified melanosomes indicate that the only motors present are kinesin-II and cytoplasmic dynein, and we believe that these motors are responsible for plus end- and minus end-directed melanosome motility, respectively. Although other motor proteins may also be present, we were unable to detect them with broadly crossreactive pan-kinesin antibodies. The in vitro velocities calculated for melanosomes traveling toward the plus and minus ends agree well with published rates of microtubule gliding for kinesin-II and dynein (23, 28). The differences in the observed rates in vitro and in the cell are probably due to drag imposed by the higher viscosity of melanophore cytoplasm. We suggest that kinesin-II and cytoplasmic dynein are the motors that power melanosome transport in vitro and are likely to be responsible for pigment motility in the cell. This hypothesis is consistent with previous studies that have attempted to identify the motors responsible for pigment transport using inhibitors and function-blocking antibodies. The dynein inhibitor sodium orthovanadate blocks pigment aggregation in detergent-permeabilized melanophores (29) as it does in our in vitro assay. Microinjection of the function-blocking polyclonal antibody, HD-kin2, raised against the kinesin motor domain, was shown to block pigment dispersion in melanophores (20). Although the initial conclusion drawn from these experiments was that kinesin is the motor for pigment dispersion, the antibody was later shown to crossreact with other members of the kinesin family and may, therefore, inhibit not only conventional kinesin, but other kinesin-like proteins (30–32). The observation that melanosomes supplemented with GTP exhibit only plus end-directed motility is also consistent with a kinesin-related protein as the plus end-directed motor, as kinesin is able to use GTP to produce force (33, 34), but dynein is not (28). These results cannot be considered as definitive proof of the involvement of kinesin-II and dynein in pigment transport, but taken together they make this hypothesis highly probable.
Melanosome motility does not require additional cytosolic factors, unlike most other systems of organelle motility studied in vitro (35–37). This demonstrates that the motors that transport the pigment granules form a stable attachment with melanosomal membranes. These observations also suggest that other soluble cofactors that are essential for motility in other systems, such as dynactin and activator II (38), are either not required by the melanosome-associated motors or remain attached throughout the purification procedure. Schnapp et al. (39) demonstrated that organelles from squid axoplasm also exhibit motility in vitro without addition of cytosolic factors. These organelles were found to contain kinesin heavy chain, but it is possible that kinesin-II may have contributed to the motility observed in this study.
Regulation of the Direction of Melanosome Movement.
There is abundant evidence that in melanophores high levels of intracellular cAMP acts through PKA to induce pigment granule dispersion (11, 40), and there is equally compelling data implicating phosphatase activity as the mechanism that triggers pigment aggregation (9, 12, 41). The molecular targets of this cyclic phosphorylation remain unidentified, however, as does an understanding of how this phosphorylation modulates motor activity. The results of our experiments demonstrate that highly purified melanosomes, free of soluble proteins, retain not only the ability to move, but also the difference in the polarity of microtubule-based movement that they have in vivo. We conclude that the final effector in the signaling pathway that regulates melanosome movement is a protein that is tightly bound to melanosomes, rather than a soluble or microtubule-associated component. As PKA is likely to be involved in the regulating the directionality of pigment granule transport, we tried to shift the polarity of movement of melanosomes from aggregated cells by treating them with the catalytic subunit of the kinase. Our preliminary results show that PKA treatment does not change the polarity of this transport, a possible indication that other soluble factors may be required for this signaling cascade (data not shown).
A characteristic feature of melanosome motility both in vivo and in vitro is its saltatory nature. This behavior suggests that the plus end- and minus end-directed melanosome-bound motors remain active during aggregation and dispersion, and the regulation of polarity is achieved by shifting the amount of time that melanosomes spend moving in either direction. The behavior of melanosomes in this respect is remarkably similar to the bidirectional microtubule gliding on coverslips coated with kinesin and dynein, described by Vale et al. (42). Moreover, transport of melanosomes via a saltatory mechanism during dispersion results in a homogeneous distribution of organelles throughout the cytoplasm, rather than a concentration at the cell periphery, which would be the expected distribution produced by unidirectional plus end-directed motility. Bidirectional organelle transport could explain the homogeneous pigment distribution in dispersed melanophores without the involvement of any other nonmicrotubule components attached to melanosomes.
The difference in the motility between melanosomes purified from aggregated and dispersed cells suggests that we can preserve the regulated state during melanosome isolation. We should, therefore, be able to purify preparative quantities of organelles from two different physiological states—one committed to aggregation and the other committed to dispersion. Direct biochemical comparison of these two organelle populations may elucidate how organelle transport in melanophores is regulated.
Acknowledgments
We thank Dr. M. Lerner for the Xenopus melanophore cell line. The following colleagues have kindly provided us with antibodies: Fatima Gyoeva (kin5-HD), Jon Scholey (K2.4), Tim Mitchison and Ken Sawin (HIPYR), Kevin Pfister (74.1), and Vincent Hearing (anti-PEP7). Dr. Birute Jakstys (Center for Electron Microscopy, University of Illinois) processed samples for electron microscopy. Video processing was performed in the Visualization Laboratory of the Beckman Institute (University of Illinois). This work was supported in part by grants from the National Institutes of Health and the National Science Foundation.
ABBREVIATIONS
- PKA
cAMP-dependent protein kinase
- IBMX
3-isobutyl-1-methyl xanthine
- AMP-PNP
5′-adenylyl-β,γ-imidodiphosphate
- DIC
differential–interference contrast
References
- 1.Hoyt M A. Curr Opin Cell Biol. 1994;6:63–68. doi: 10.1016/0955-0674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 2.Cole N B, Lippincott-Schwartz J. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 3.Lippincott-Schwartz L. Trends Cell Biol. 1993;3:81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- 4.Coy D L, Howard J. Curr Opin Neurobiol. 1994;4:662–667. doi: 10.1016/0959-4388(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 5.Vernos I, Karsenti E. Curr Opin Cell Biol. 1996;8:4–9. doi: 10.1016/s0955-0674(96)80041-x. [DOI] [PubMed] [Google Scholar]
- 6.Haimo L T, Thaler C D. BioEssays. 1994;16:727–733. [Google Scholar]
- 7.Euteneuer U, McIntosh J R. Proc Natl Acad Sci USA. 1981;78:372–376. doi: 10.1073/pnas.78.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rozdzial M M, Haimo L T. J Cell Biol. 1986;103:2755–2764. doi: 10.1083/jcb.103.6.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sammak P J, Adams S R, Harootunian A T, Schliwa M, Tsien R Y. J Cell Biol. 1992;117:57–72. doi: 10.1083/jcb.117.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniolos A, Lerner A B, Lerner M R. Pigment Cell Res. 1990;3:38–43. doi: 10.1111/j.1600-0749.1990.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 11.Rozdzial M M, Haimo L T. Cell. 1986;47:1061–1070. doi: 10.1016/0092-8674(86)90821-4. [DOI] [PubMed] [Google Scholar]
- 12.Cozzi B, Rollag M D. Pigment Cell Res. 1992;5:148–154. doi: 10.1111/j.1600-0749.1992.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 13.Weingarten R C, Lockwood A H, Huo S Y, Kirschner M W. Proc Natl Acad Sci USA. 1975;73:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons I R, Lee-Eiford A, Mocz G, Phillipson C A, Tang W J, Gibbons B H. J Biol Chem. 1987;262:2780–2786. [PubMed] [Google Scholar]
- 15.Weetall H H. Methods Enzymol. 1976;44:134–148. doi: 10.1016/s0076-6879(76)44012-0. [DOI] [PubMed] [Google Scholar]
- 16.Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Stefen P, Wordeman L, Mitchison T. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez M, Tsukamoto K, Hearing V J. J Biol Chem. 1991;266:1147–1156. [PubMed] [Google Scholar]
- 20.Rodionov V I, Gyoeva F K, Gelfand V I. Proc Natl Acad Sci USA. 1991;88:4956–4960. doi: 10.1073/pnas.88.11.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole D G, Cande W Z, Baskin R J, Skoufias D A, Hogan C J, Scholey J M. J Cell Sci. 1992;101:291–301. doi: 10.1242/jcs.101.2.291. [DOI] [PubMed] [Google Scholar]
- 22.Sawin K E, Mitchison T J, Wordeman L G. J Cell Sci. 1992;101:303–313. doi: 10.1242/jcs.101.2.303. [DOI] [PubMed] [Google Scholar]
- 23.Cole D G, Chinn S W, Wedaman K P, Hall K, Vuong T, Scholey J M. Nature (London) 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- 24.Dillman J F, Pfister K K. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearing V, Tsukamoto K. FASEB J. 1991;5:2902–2909. [PubMed] [Google Scholar]
- 26.Scholey J M. J Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan V. J Cell Biol. 1995;128:879–891. doi: 10.1083/jcb.128.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paschal B M, Shpetner H S, Vallee R B. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark T G, Rosenbaum J L. Proc Natl Acad Sci USA. 1982;79:4655–4659. doi: 10.1073/pnas.79.15.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright B D, Terasaki M, Scholey J M. J Cell Biol. 1993;123:681–689. doi: 10.1083/jcb.123.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middleton K, Carbon J. Proc Natl Acad Sci USA. 1994;91:7212–7216. doi: 10.1073/pnas.91.15.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombillo V A, Nislow C, Yen T J, Gelfand V I, McIntosh J R. J Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter M E, Scholey J M, Stemple D L, Vigers G P, Vale R D, Sheetz M P, McIntosh J R. J Biol Chem. 1987;262:2794–2802. [PubMed] [Google Scholar]
- 34.Shimizu T, Toyoshima Y Y, Vale R D. In: Motility Assays for Motor Proteins. Scholey J M, editor. Vol. 39. San Diego: Academic; 1993. pp. 167–190. [Google Scholar]
- 35.Dabora S L, Sheetz M P. Cell Motil Cytoskeleton. 1988;10:482–495. doi: 10.1002/cm.970100405. [DOI] [PubMed] [Google Scholar]
- 36.Schroer T A, Schnapp B J, Reese T S, Sheetz M P. J Cell Biol. 1988;107:1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allan V J, Vale R D. J Cell Biol. 1991;113:347–359. doi: 10.1083/jcb.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroer T A, Sheetz M P. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnapp B J, Reese T S, Bechtold R. J Cell Biol. 1992;119:389–399. doi: 10.1083/jcb.119.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClintock T, Rising J, Lerner M. J Cell Physiol. 1996;167:1–7. doi: 10.1002/(SICI)1097-4652(199604)167:1<1::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 41.Thaler C D, Haimo L T. J Cell Biol. 1990;111:1939–1948. doi: 10.1083/jcb.111.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vale R D, Malik F, Brown D. J Cell Biol. 1992;119:1589–1596. doi: 10.1083/jcb.119.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]