Abstract
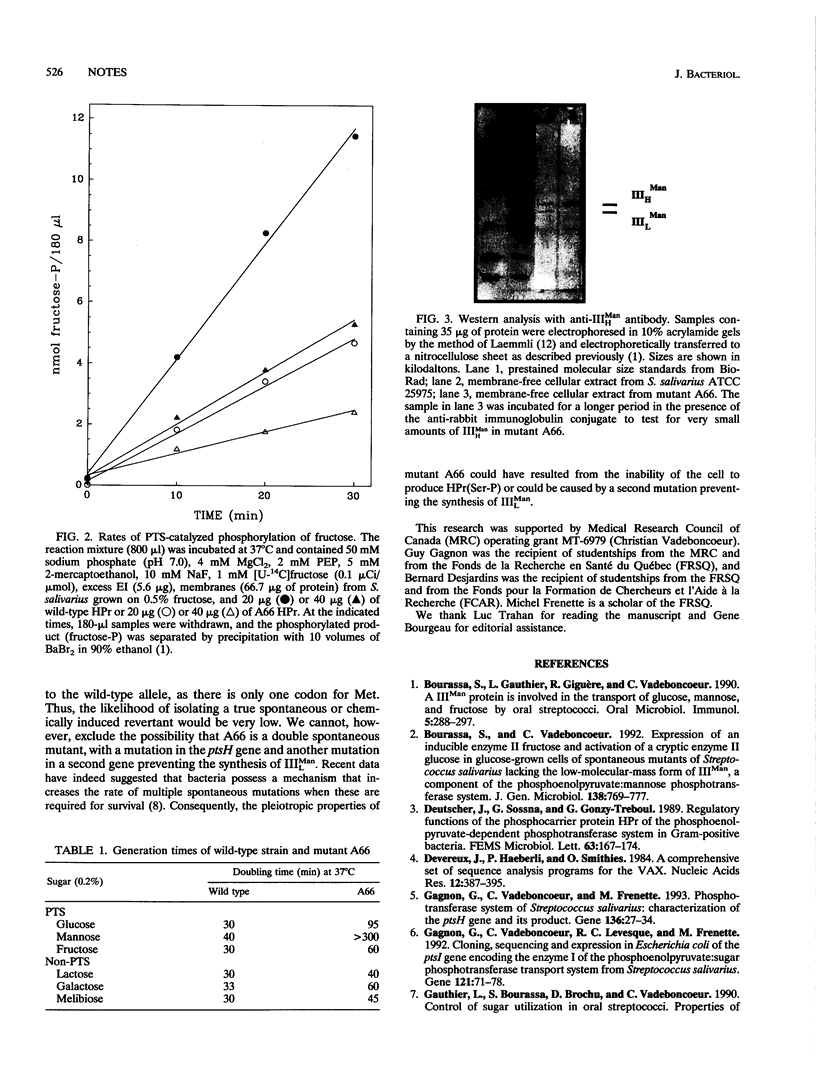
HPr is a protein of the phosphoenolpyruvate:sugar phosphotransferase system (PTS) that participates in the concomitant transport and phosphorylation of sugars in bacteria. In gram-positive bacteria, HPr is also reversibly phosphorylated at a seryl residue at position 46 (Ser-46) by a metabolite-activated ATP-dependent kinase and a Pi-dependent HPr(Ser-P) phosphatase. We report in this article the isolation of a spontaneous mutant (mutant A66) from a streptococcus (Streptococcus salivarius) in which the methionine at position 48 (Met-48) in the protein HPr has been replaced by a valine (Val). The mutation inhibited the phosphorylation of HPr on Ser-46 by the ATP-dependent kinase but did not prevent phosphorylation of HPr by enzyme I or the phosphorylation of enzyme II complexes by HPr(His-P). The results, however, suggested that replacement of Met-48 by Val decreased the affinity of enzyme I for HPr or the affinity of enzyme II proteins for HPr(His-P) or both. Characterization of mutant A66 demonstrated that it has pleiotropic properties, including the lack of IIILman, a specific protein of the mannose PTS; decreased levels of HPr; derepression of some cytoplasmic proteins; reduced growth on PTS as well as on non-PTS sugars; and aberrant growth in medium containing a mixture of sugars.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourassa S., Gauthier L., Giguère R., Vadeboncoeur C. A IIIman protein is involved in the transport of glucose, mannose and fructose by oral streptococci. Oral Microbiol Immunol. 1990 Oct;5(5):288–297. doi: 10.1111/j.1399-302x.1990.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Bourassa S., Vadeboncoeur C. Expression of an inducible enzyme II fructose and activation of a cryptic enzyme II glucose in glucose-grown cells of spontaneous mutants of Streptococcus salivarius lacking the low-molecular-mass form of IIIman, a component of the phosphoenolpyruvate:mannose phosphotransferase system. J Gen Microbiol. 1992 Apr;138(4):769–777. doi: 10.1099/00221287-138-4-769. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Sossna G., Gonzy-Treboul G. Regulatory functions of the phosphocarrier protein HPr of the phosphoenol pyruvate-dependent phosphotransferase system in gram-positive bacteria. FEMS Microbiol Rev. 1989 Jun;5(1-2):167–174. doi: 10.1016/0168-6445(89)90021-1. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon G., Vadeboncoeur C., Frenette M. Phosphotransferase system of Streptococcus salivarius: characterization of the ptsH gene and its product. Gene. 1993 Dec 22;136(1-2):27–34. doi: 10.1016/0378-1119(93)90443-7. [DOI] [PubMed] [Google Scholar]
- Gagnon G., Vadeboncoeur C., Levesque R. C., Frenette M. Cloning, sequencing and expression in Escherichia coli of the ptsI gene encoding enzyme I of the phosphoenolpyruvate:sugar phosphotransferase transport system from Streptococcus salivarius. Gene. 1992 Nov 2;121(1):71–78. doi: 10.1016/0378-1119(92)90163-j. [DOI] [PubMed] [Google Scholar]
- Gauthier L., Bourassa S., Brochu D., Vadeboncoeur C. Control of sugar utilization in oral streptococci. Properties of phenotypically distinct 2-deoxyglucose-resistant mutants of Streptococcus salivarius. Oral Microbiol Immunol. 1990 Dec;5(6):352–359. doi: 10.1111/j.1399-302x.1990.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations: mutations involving base substitutions. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5882–5886. doi: 10.1073/pnas.88.13.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Gauthier L., Desjardins B., Vadeboncoeur C. Concentration-dependent repression of the soluble and membrane components of the Streptococcus mutans phosphoenolpyruvate: sugar phosphotransferase system by glucose. J Bacteriol. 1989 Jun;171(6):2942–2948. doi: 10.1128/jb.171.6.2942-2948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg O., Reddy P., Sutrina S., Saier M. H., Jr, Reizer J., Kapadia G. Structure of the histidine-containing phosphocarrier protein HPr from Bacillus subtilis at 2.0-A resolution. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2499–2503. doi: 10.1073/pnas.89.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Mimura C. S., Poy F., Jacobson G. R. ATP-dependent protein kinase activities in the oral pathogen Streptococcus mutans. J Cell Biochem. 1987 Mar;33(3):161–171. doi: 10.1002/jcb.240330303. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Deutscher J., Saier M. H., Jr Metabolite-sensitive, ATP-dependent, protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system in gram-positive bacteria. Biochimie. 1989 Sep-Oct;71(9-10):989–996. doi: 10.1016/0300-9084(89)90102-8. [DOI] [PubMed] [Google Scholar]
- Reizer J., Hoischen C., Reizer A., Pham T. N., Saier M. H., Jr Sequence analyses and evolutionary relationships among the energy-coupling proteins Enzyme I and HPr of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Protein Sci. 1993 Apr;2(4):506–521. doi: 10.1002/pro.5560020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Peterkofsky A., Romano A. H. Evidence for the presence of heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking phosphoenolpyruvate:glycose phosphotransferase activity. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Romano A. H., Deutscher J. The role of phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, in the regulation of carbon metabolism in gram-positive bacteria. J Cell Biochem. 1993 Jan;51(1):19–24. doi: 10.1002/jcb.240510105. [DOI] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Saier M. H., Stewart G. C., Peterkofsky A., Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989 Jul;8(7):2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Reizer J. Proposed uniform nomenclature for the proteins and protein domains of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Bacteriol. 1992 Mar;174(5):1433–1438. doi: 10.1128/jb.174.5.1433-1438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Mattoo R. L., Erickson E., Vadeboncoeur C. Phosphoproteins and the phosphoenolpyruvate:sugar phosphotransferase system of Streptococcus salivarius. Detection of two different ATP-dependent phosphorylations of the phosphocarrier protein HPr. Can J Microbiol. 1986 Apr;32(4):310–318. doi: 10.1139/m86-062. [DOI] [PubMed] [Google Scholar]