Abstract
Background and purpose:
Macrophages in atherosclerotic plaques have a tremendous impact on atherogenesis and plaque destabilization. We previously demonstrated that treatment of plaques in cholesterol-fed rabbits with the nitric oxide (NO) donor molsidomine preferentially eliminates macrophages, thereby favouring features of plaque stability. In this study, we investigated the underlying mechanism.
Experimental approach:
Macrophages and smooth muscle cells (SMCs) were treated in vitro with the NO donors, spermine NONOate or S-nitroso-N-acetylpenicillamine (SNAP) as well as with the well-known endoplasmic reticulum (ER) stress inducers thapsigargin, tunicamycin, dithiothreitol or brefeldin A. Cell viability was analysed by Neutral Red viability assays. Cleavage of caspase-3, DNA fragmentation and ultrastructural changes were examined to characterize the type of macrophage death. Induction of ER stress was evaluated by measuring C/EBP homologous protein (CHOP) expression, phosphorylation of eukaryotic initiation factor 2α (eIF2a), splicing of X-box binding protein 1 (XBP1) and inhibition of protein synthesis.
Key results:
Macrophages and SMCs treated with spermine NONOate or SNAP showed several signs of ER stress, including upregulation of CHOP expression, hyperphosphorylation of eIF2α, inhibition of de novo protein synthesis and splicing of XBP1 mRNA. These effects were similar in macrophages and SMCs, yet only macrophages underwent apoptosis. Plaques from molsidomine-treated atherosclerotic rabbits showed a 2.7-fold increase in CHOP expression as compared to placebo. Beside NO, selective induction of macrophage death could be initiated with thapsigargin and tunicamycin.
Conclusions and implications:
Induction of ER stress explains selective depletion of macrophages in atherosclerotic plaques by a NO donor, probably via inhibition of protein synthesis.
Keywords: nitric oxide, atherosclerosis, apoptosis, endoplasmic reticulum stress, macrophages, smooth muscle cells, thapsigargin
Introduction
Atherosclerotic plaque destabilization and rupture are thought to account for most acute coronary syndromes, suggesting that plaque instability rather than plaque progression is a major target for new therapies (Gutstein and Fuster, 1999). Unstable, rupture-prone atherosclerotic plaques are defined as lesions with a thin fibrous cap containing few smooth muscle cells (SMCs) and a large lipid core surrounded by numerous foam cells of macrophage origin. Plaque destabilization may start by external factors such as increased blood pressure and shear stress (Slager et al., 2005) and/or by factors within the atherosclerotic plaque, in particular inflammation (Libby, 2002), intraplaque haemorrhage (Kockx et al., 2003; Kolodgie et al., 2003) and the induction of SMC death (Clarke et al., 2006). Pharmacological agents such as lipid-lowering agents, β-adrenoceptor antagonists, angiotensin-converting enzyme inhibitors and antioxidants may target plaque destabilization and have been shown to reduce the incidence of acute coronary syndromes (Rabbani and Topol, 1999). Yet, cardiovascular disease resulting from atherosclerosis and thrombosis remains a major cause of death and disability among adults in Western countries. Because macrophages and their secretory products have a tremendous impact on plaque destabilization (Libby, 2002), there is now a growing interest for therapeutic strategies that may lead to a selective, clean and safe removal of macrophages within the atherosclerotic plaque (Martinet et al., 2007). However, the majority of methodologies that have been developed so far reduce peripheral blood monocytes and tissue macrophages in a nonselective way or deal with serious adverse effects. In a previous study, treatment of atherosclerotic rabbits with the nitric oxide (NO) donor molsidomine cleared subendothelial macrophages and led to plaques that consisted predominantly of SMCs and collagen fibres (De Meyer et al., 2003). Since the latter give tensile strength to the plaque, these changes represent some features of stable human atherosclerotic plaques. Molsidomine is a prodrug that acts via the metabolite 3-morpholino-sydnonimine (SIN-1). In vitro, SIN-1 simultaneously generates NO and superoxide (Feelisch et al., 1989), but in vivo, endogenous oxidizing agents other than molecular oxygen, such as ferric haemproteins, can oxidize SIN-1, resulting in the release of NO without concomitant formation of superoxide (Singh et al., 1999). The precise mechanism underlying selective depletion of macrophages in molsidomine-treated plaques is presently unclear although there are several possibilities. NO donors can decrease the expression of adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells so that molsidomine might attenuate macrophage influx into the arterial wall. Because VCAM-1 expression was not affected in plaques from molsidomine-treated rabbits as compared to placebo (De Meyer et al., 2003), this possibility seems unlikely. Increased cell death of macrophages provides another explanation. Indeed, it has been shown that NO can induce macrophage apoptosis via upregulation of the proapoptotic protein p53 (Messmer et al., 1994) or induction of endoplasmic reticulum (ER) stress (Gotoh and Mori, 2006). In the present study, we provide evidence that NO-induced ER stress can induce macrophage cell death without affecting SMC viability.
Methods
Cell culture
The murine macrophage cell line J774A.1 was grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 50 μg ml−1 gentamicin, 20 U ml−1 polymyxin B and 10% fetal bovine serum (FBS). Alternatively, peritoneal macrophages were isolated 5 days after injection of 2 ml Brewer's thioglycolate medium (Sigma-Aldrich, St Louis, MO, USA) into the peritoneal cavity of C57BL/6 mice as reported previously (McCarron et al., 1984). Primary macrophages were added to culture flasks and allowed to adhere for 2 h at 37°C. Nonadherent cells were removed by three washes of warm medium. Adherent cells were >99% macrophages as assessed by immunocytochemical detection of the macrophage marker F4/80 (anti-F4/80, clone Cl:A3-1; Serotec, Oxford, UK). SMCs were isolated from mouse or rabbit aorta by collagenase type 2 (Worthington, Lakewood, NJ, USA) and elastase (Sigma-Aldrich) digestion (60–90 min at 37°C) at 300 and 5 U ml−1 final concentration, respectively, and cultured in Ham F10 medium (Invitrogen) supplemented with 10% FBS and antibiotics. C2C12 myoblasts were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and antibiotics. Stock solutions (35 in 10 mM NaOH) of spermine NONOate (Sigma-Aldrich) were freshly prepared or stored at −80°C for up to 1 month. Stock solutions (50 mM) of S-nitroso-N-acetylpenicillamine (SNAP) were prepared by combining equal volumes of N-acetyl-D-penicillamine (19 mg ml−1 in 100% ethanol) and NaNO2 (7 mg ml−1 in RNase-free water). The mixture was acidified with 50 μl hydrochloric acid (19% (v/v)) per 1 ml SNAP solution and incubated for at least 30 min at 4°C before use. Stock solutions of SNAP were prepared immediately before administration.
Evaluation of cell viability was based on the incorporation of the supravital dye Neutral Red by viable cells (Lowik et al., 1993). For DNA fragmentation assays, cultured cells (106) were lysed in 0.5 ml hypotonic lysis buffer (10 mM Tris, 1 mM EDTA, 0.2% Triton X-100) supplemented with 250 μg proteinase K. Lysates were incubated for 1 h at 50°C, then supplemented with 5 μl volumes of DNase-free RNase A (2 mg ml−1) and incubated for an additional hour at 37°C. The samples were precipitated overnight with 1/10 volume of 3 M sodium acetate and one volume of isopropanol. DNA pellets were air-dried and dissolved in TE buffer (10 mM Tris, 1 mM EDTA, pH 7.4). After electrophoresis in 2% agarose, DNA laddering was visualized under UV light by staining the agarose gel with ethidium bromide. To examine de novo protein synthesis, cells were pulse-labelled for 1 h at 37°C with 5 μCi Pro-mix L-[35S] in vitro cell labelling mix (GE Healthcare, Little Chalfont, UK) in cysteine/methionine-free DMEM (Invitrogen). After homogenization of cells in hypotonic lysis buffer, labelled proteins were precipitated with 10% trichloroacetic acid, resuspended in 0.2 N NaOH and measured by liquid scintillation counting.
Total RNA was isolated from cultured cells using the Absolutely RNA Microprep Kit (Stratagene, La Jolla, CA, USA). Alternative splicing of XBP1 mRNA was examined by reverse transcription (RT)–PCR using XBP1-specific primers (5′-GATCCTGACGAGGTTCCAGAGGTG-3′ (forward primer) and 5′-GAGTCAGAGTCCATGGGAAGATGTTCTG-3′ (reverse primer)) and the Superscript One-Step RT–PCR Kit (Invitrogen). Thermocycling parameters were as follows: reverse transcription at 50°C for 30 min, denaturation at 94°C for 2 min and 40 cycles consisting of incubations at 94°C for 15 s, 60°C for 30 s and 72°C for 30 s. PCR products were analysed on 4% E-gels (Invitrogen).
Isolation of low-density lipoprotein
Human blood samples from fasting normolipidaemic healthy volunteers were centrifuged and plasma was adjusted to a density of 1.24 g ml−1 with KBr (381.6 mg ml−1). A gradient was formed by layering the density-adjusted plasma underneath phosphate-buffered saline (PBS). Plasma lipoproteins were separated by ultracentrifugation in a Sorvall TFT65.13 rotor (189 280 g, 5 h). Low-density lipoprotein (LDL) was isolated and dialyzed against EDTA-containing PBS to remove remaining KBr. Aggregated LDL (agLDL) was prepared by vortexing LDL solution for 2 min.
Western blot analysis
Cultured cells were lysed in an appropriate volume of Laemmli sample buffer (Bio-Rad, Richmond, CA, USA). Cell lysates were then heat-denatured for 4 min in boiling water and loaded on a sodium dodecyl sulphate (SDS) polyacrylamide gel. After electrophoresis, proteins were transferred to an Immobilon-P Transfer Membrane (Millipore, Bedford, MA, USA) according to standard procedures. Membranes were blocked in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) and 5% nonfat dry milk (Bio-Rad) for 1 h. After blocking, membranes were probed overnight at 4°C with primary antibodies in antibody dilution buffer (TBS-T containing 1% nonfat dry milk), followed by 1 h incubation with secondary antibody at room temperature. Antibody detection was accomplished with SuperSignal West Pico or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA) using a Lumi-Imager (Roche Diagnostics, Mannheim, Germany).
The following mouse monoclonal antibodies were used: anti-p53 (clone Pab 240) from BD Pharmingen (San Jose, CA, USA), anti-caspase-3 (clone 19) from BD Transduction Laboratories (Lexington, KY, USA), anti-GADD153/CHOP (clone B-3) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-β-actin (clone AC-15) from Sigma-Aldrich. Polyclonal rabbit antibodies that were used include anti-cleaved caspase-3, anti-eukaryotic initiation factor 2α (eIF2α) and anti-phospho-eIF2α (Ser51) from Cell Signaling Technology (Beverly, MA, USA). Peroxidase-conjugated secondary antibodies were purchased from DakoCytomation (Glostrup, Denmark).
Real-time quantitative RT–PCR
cDNA was prepared from cultured cells using the Fastlane Cell cDNA kit (Qiagen, Venlo, The Netherlands). TaqMan gene-expression assays for CHOP (assay Id: Mm00492097_m1, Applied Biosystems, Foster City, CA, USA) were then performed in duplicate on an ABIPrism 7300 sequence detector system (Applied Biosystems) in 25 μl reaction volumes containing 1 × Universal PCR Master Mix (Applied Biosystems). The parameters for PCR amplification were 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative expression of mRNA species was calculated using the comparative threshold cycle method. All data were controlled for quantity of cDNA input by performing measurements on the endogenous reference gene β-actin (assay Id: Mm00607939_s1, Applied Biosystems).
Animals
All animal procedures were approved by the Ethical Committee of the University of Antwerp. Male New Zealand white rabbits were fed a 0.3% cholesterol diet for 20 weeks to induce atherosclerotic plaques in the aorta. Subsequently, the animals received a normal diet (150 g chow per day) or a normal diet plus molsidomine (1 mg kg−1 day−1) in the drinking water (100 ml tap water twice a day) for 4 weeks. Frozen sections of plaques from the ascending aorta were homogenized in Laemmli sample buffer and used for western blot detection of CHOP. After normalizing for sample load using β-actin as an internal standard, expression of CHOP in each plaque section was calculated.
Electron microscopy
Tissue samples were fixed in 0.1 M sodium cacodylate-buffered (pH 7.4) 2.5% glutaraldehyde solution for 2 h, then rinsed (3 × 10 min) in 0.1 M sodium cacodylate-buffered (pH 7.4) 7.5% saccharose and postfixed in 1% OsO4 solution for 1 h. After dehydration in an ethanol gradient (70% ethanol (20 min), 96% ethanol (20 min)), 100% ethanol (2 × 20 min)), samples were embedded in Durcupan ACM. Ultrathin sections were stained with uranyl acetate and lead citrate. Sections were examined in a Philips CM 10 microscope at 80 kV.
Statistical analysis
Values are presented as mean±s.e.mean. Differences between means were assessed with one-way ANOVA and Dunnett's test or by Student's t-test, as appropriate. Probability levels less than 0.05 were considered statistically significant. All analyses were performed using SPSS 12.0 software (Chicago, IL, USA).
Results
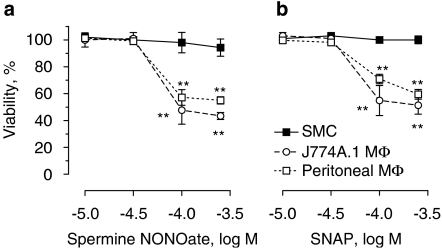
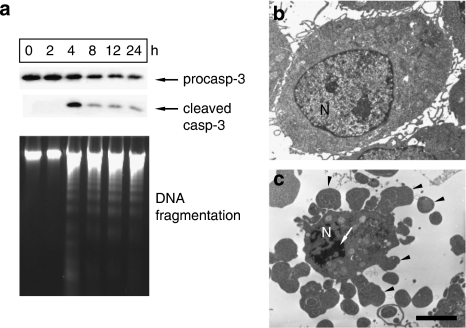
J774A.1 macrophages, thioglycolate-elicited peritoneal macrophages and mouse or rabbit aortic SMCs were treated in vitro with the NO donors, spermine NONOate or SNAP (10−5–3 × 10−4 M). The active metabolite of molsidomine, SIN-1, was not used here because this compound generates NO and superoxide (Feelisch et al., 1989) and thus acts predominantly as a peroxynitrite donor (not as a NO donor) in vitro. Although SMCs survived treatment, viability of J774A.1 cells and peritoneal macrophages decreased with approximately 50% (Figure 1). Moreover, C2C12 myoblasts were highly resistant to NO-induced cell death as compared to macrophages (data not shown), suggesting a cell type-specific initiation of cell death. Because J774A.1 cells and peritoneal macrophages reacted similarly to spermine NONOate and SNAP, the type of macrophage cell death as well as the underlying mechanism was further examined in J774A.1 cells. Death of J774A.1 cells induced by spermine NONOate was characterized by cleavage of caspase-3 and internucleosomal DNA fragmentation (Figure 2a), typical of apoptosis. Moreover, several morphological changes characteristic of apoptosis such as cellular shrinkage, chromatin condensation, the persistence of a continuous plasma membrane and the formation of protuberances on the cell surface that may precede the shedding of apoptotic bodies could be detected (Figures 2b and c). Despite previous findings showing that NO-induced apoptosis can be mediated by the DNA damage pathway involving accumulation of p53 (Messmer et al., 1994), only DNA-damaging agents such as camptothecin but not spermine NONOate upregulated p53 protein expression in J774A.1 cells (not shown).
Figure 1.
Effect of the nitric oxide donors spermine NONOate and S-nitroso-N-acetylpenicillamine (SNAP) on viability of J774A.1 macrophages (MΦ), thioglycolate elicited peritoneal macrophages and smooth muscle cells (SMCs). Cells were exposed to various concentrations of spermine NONOate (a) or SNAP (b) in serum-containing medium for 24 h. Cell death was examined by Neutral Red viability assays. Results represent the mean±s.e.mean of three independent experiments. **P<0.01 versus untreated cells (ANOVA, followed by Dunnett's test).
Figure 2.
Characterization of spermine NONOate-induced cell death in J774A.1 macrophages. Cells were treated with 300 μM spermine NONOate for up to 24 h. Cleavage of procaspase-3 (procasp-3) and internucleosomal DNA fragmentation was analysed using western blotting (top) and agarose gel electrophoresis (bottom), respectively (a). Ultrastructural features of J774A.1 cells before (b) and after treatment with 300 μM spermine NONOate for 3 h (c) were analysed by electron microscopy. Spermine NONOate-treated cells were characterized by chromatin condensation (arrow) and blebbing of the plasma membrane (arrowheads). N indicates nucleus. Scale bar=2 μm. Results are representative images of three independent experiments.
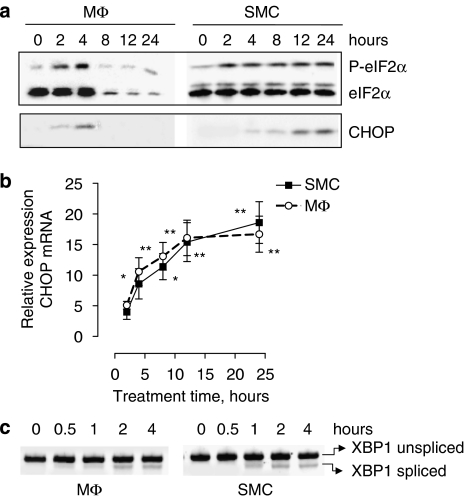
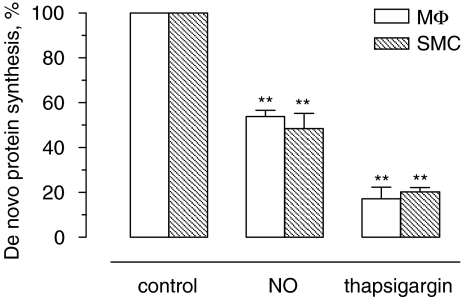
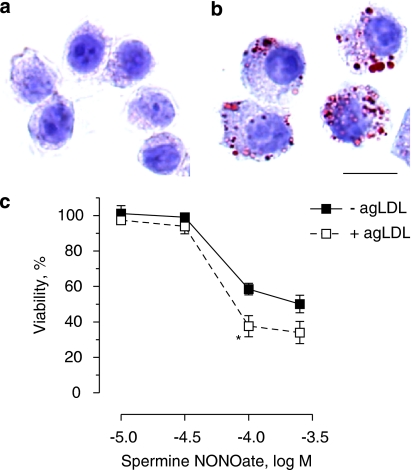
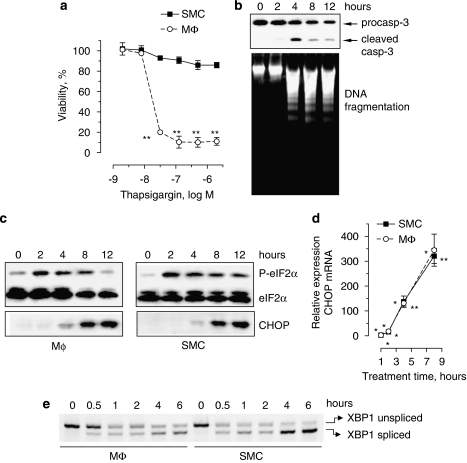
However, treatment of J774A.1 cells with spermine NONOate triggered ER stress, as shown by hyperphosphorylation of eIF2α (Figure 3a), upregulation of C/EBP homologous protein (CHOP) mRNA (Figure 3b), mRNA splicing of the ER stress mediator X-box-binding protein 1 (XBP1; Figure 3c) and inhibition of de novo protein synthesis (Figure 4). The induction of ER stress was similar in J774A.1 macrophages and SMCs. CHOP protein accumulation was detectable after 4 h of treatment, but decreased in macrophages at later time points (Figure 3a), most likely due to initiation of cell death. For similar reasons, expression of total eIF2α and thus also phosphorylated (Ser51) eIF2α declined in macrophages after 8 h of treatment. As observed in the in vitro experiments, atherosclerotic plaques from cholesterol-fed rabbits that received the NO donor molsidomine in their drinking water contained higher levels (2.7-fold) of CHOP protein as compared to plaques from nontreated control rabbits (1.82±0.44 arbitrary unit (AU; n=9) versus 0.67±0.11 AU (n=8), P<0.05 l (unpaired Student's t-test)). Because uptake of modified LDL by macrophages and formation of macrophage-derived foam cells is a hallmark of atherosclerosis, J774A.1 cells were incubated in an additional series of experiments with agLDL to mimic foam cell formation (Figures 5a and b). Lipid-laden J774A.1 cells were more sensitive to spermine NONOate-induced cell death as compared to controls (Figure 5c).
Figure 3.
Induction of endoplasmic reticulum (ER) stress in J774A.1 macrophages (MΦ) and smooth muscle cells (SMCs) treated with 300 μM spermine NONOate for up to 24 h. Expression of the ER stress protein C/EBP homologous protein (CHOP) as well as phosphorylation of eukaryotic initiation factor 2α (P-elF2α) was analysed by western blotting (a). Relative expression of CHOP mRNA (b) and splicing of X-box-binding protein 1 (XBP1) mRNA (c) was examined via real-time quantitative reverse transcription (RT)–PCR or conventional RT–PCR, respectively. *P<0.05; **P<0.01 versus untreated cells (ANOVA, followed by Dunnett's test). Results are representative of three independent experiments.
Figure 4.
Inhibition of de novo protein synthesis in J774A.1 macrophages (MΦ) and smooth muscle cells (SMCs) after treatment with spermine NONOate (NO) or thapsigargin. Cells were treated with 300 μM spermine NONOate or 1 μM thapsigargin for 1 h in serum-containing medium and then pulse-labelled for 1 h with L-35S-methionine/cysteine. Labelled proteins were measured by scintillation counting. Results represent the mean±s.e.mean of three independent experiments. **P<0.01 versus control (ANOVA, followed by Dunnett's test).
Figure 5.
Spermine NONOate-induced cell death in J774A.1 macrophages after phagocytosis of aggregated low-density lipoprotein (agLDL). (a and b) Oil Red O staining of J774A.1 cells grown in the absence (a) or presence (b) of 200 μg/ml agLDL for 20 h. Scale bar=10 μm. (c) Viability of agLDL-laden and control J774A.1 cells after exposure to different concentrations of spermine NONOate. Results represent the mean±s.e.mean of three independent experiments. *P<0.05 versus cells without agLDL (unpaired Student's t-test).
HMG-CoA reductase inhibitors (statins) have beneficial effects on the architecture of atherosclerotic lesions, including a reduction in the number of plaque macrophages (Libby and Aikawa, 2003). Apart from their well-described lipid-lowering and anti-inflammatory effects, statins are able to induce macrophage death (Kim et al., 2006; Liang et al., 2006). For example, induction of J774A.1 macrophage death was observed after treatment with 100 nM fluvastatin (V Croons, unpublished results). Incubation of J774A.1 macrophages with sublethal levels of fluvastatin (10 nM) did not affect spermine NONOate-induced cell death (data not shown).
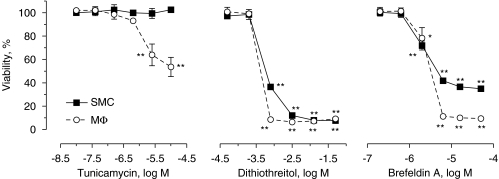
To further determine whether ER stress selectively induced macrophage apoptosis, J774A.1 cells and SMCs were treated with different, well-known ER stress inducers. Thapsigargin induced ER stress both in macrophages and SMCs (Figure 6), yet only macrophages underwent massive apoptosis (Figures 6a and b). ER stress in thapsigargin-treated cells was characterized by upregulation of CHOP (Figures 6c and d), hyperphosphorylation of eIF2α (Figure 6c), alternative mRNA splicing of XBP1 (Figure 6e) and inhibition of de novo protein synthesis (Figure 4). Furthermore, macrophages were more sensitive to the N-glycosylation inhibitor and ER stress inducer tunicamycin as compared to SMCs (Figure 7). Selective induction of cell death by other ER stress inducers such as dithiothreitol or brefeldin A did not occur or was not clear-cut (Figure 7), possibly due to additional effects unrelated to ER stress.
Figure 6.
Selective induction of J774A.1 macrophage cell (MΦ) death by the endoplasmic reticulum (ER) stress inducer thapsigargin. (a) Viability of MΦ and smooth muscle cells (SMCs) after exposure to different concentrations of thapsigargin for 24 h. **P<0.01 versus untreated cells (ANOVA, followed by Dunnett's test). (b–e) Cells were treated with 1 μm thapsigargin for the time indicated. Thapsigargin-induced J774A.1 cell death was characterized by cleavage of procaspase-3 (procasp-3) and internucleosomal DNA fragmentation (b). Western blot of eukaryotic initiation factor 2α (eIF2α) phosphorylation and C/EBP homologous protein (CHOP) expression (c), real-time reverse transcription (RT)–PCR analysis of CHOP mRNA (d), *P<0.05; **P<0.01 versus untreated cells (one-sample t-test), as well as splicing of X-box-binding protein 1 (XBP1) mRNA (e) was performed to document induction of ER stress. Results are representative of three independent experiments.
Figure 7.
Viability of J774A.1 macrophages (MΦ) and smooth muscle cells (SMCs) after exposure to different concentrations of the endoplasmic reticulum stress inducers tunicamycin, dithiothreitol and brefeldin A for 24 h in serum-containing medium. Results represent the mean±s.e.mean of three independent experiments. *P<0.05; **P<0.01 versus untreated cells (ANOVA, followed by Dunnett's test).
Discussion
Treatment of plaques in cholesterol-fed rabbits with the NO donor molsidomine exerts several beneficial effects on plaque structure and stability, including the formation of a large subendothelial macrophage-free layer consisting of SMCs and extracellular matrix as well as the normalization of superoxide production and ecSOD mRNA expression (De Meyer et al., 2003). In this study, we report that the selective depletion of macrophages in molsidomine-treated plaques is mediated, at least in part, by the induction of ER stress.
The ER is an organelle in which secretory or cell-surface proteins, as well as resident proteins of the secretory pathway are synthesized, folded and modified (Berridge, 2002). To carry out these functions efficiently, the ER relies on numerous resident chaperone proteins, a high level of Ca2+ and an oxidative environment. Yet, this organelle remains highly sensitive to alterations in Ca2+ homeostasis and perturbation of its environment. Indeed, several lines of evidence indicate that NO can disrupt ER function resulting in ER stress (Gotoh and Mori, 2006). To survive this type of stress, the ER responds by triggering the unfolded protein response (UPR; Rutkowski and Kaufman, 2004). Three ER-resident transmembrane proteins have been identified as proximal sensors during the UPR: the PKR-like ER kinase (PERK), the transcription factor ATF6 and the endonuclease Ire1 (endoplasmic reticulum-to-nucleus signalling 1). Each of these proteins is constitutively expressed in all cells and bound to the ER-resident molecular chaperone BiP (immunoglobulin heavy-chain-binding protein). When the ER is stressed, PERK releases from BiP and transiently attenuates translation by phosphorylating initiation factor 2α (eIF2α), thereby limiting protein load in the stressed ER. ATF6 drives the transcriptional upregulation of many ER-resident proteins and folding assistants. Ire1 activates XBP1 via splicing, which in turn induces transcription of factors that facilitate ER-associated degradation. In the present study, macrophages and SMCs treated with the NO donors spermine NONOate or SNAP revealed several signs of ER stress including hyperphosphorylation of eIF2α, inhibition of de novo protein synthesis and splicing of XBP1 mRNA. These effects were similar in macrophages and SMCs, yet only macrophages underwent apoptosis. Interestingly, selective induction of macrophage death could also be initiated with well-known ER stress inducers such as the Ca2+ homeostasis disruptor thapsigargin and the N-linked glycosylation inhibitor tunicamycin, reinforcing the finding that induction of ER stress during treatment with NO donors initiates selective macrophage death.
Accumulation of free cholesterol in macrophages is important for the progression of atherosclerosis and stimulates ER stress and UPR activation (Tabas, 2002). As a consequence, ER stress is already present in macrophages in early stages of atherosclerosis (Zhou et al., 2005). Because UPR is also activated in intimal macrophages before significant accumulation of intracellular free cholesterol (Zhou et al., 2005), ER stress stimuli unrelated to free cholesterol, such as oxidative stress, seem to be present in developing plaques. There is, however, no substantial evidence of apoptotic cell death in macrophage foam cells in early lesions, supporting the concept that activation of additional cellular mediators and/or pathways is required for macrophage apoptosis (DeVries-Seimon et al., 2005). Because only prolonged or severe ER stress results in apoptotic cell death (Okada et al., 2004), it is conceivable that the majority of macrophages in atherosclerotic plaques are already sensitized to undergo ER stress-induced apoptosis, as shown in the present study for J774A.1 cells exposed to agLDL, and that administration of ER stress inducers, such as NO donors, can selectively provoke macrophage apoptosis, even at low concentrations. This occurs in a safe way, that is, without affecting the viability of circulating monocytes/macrophages.
Multiple proapoptotic signalling pathways emanate from the ER (Rutkowski and Kaufman, 2004). Some of the molecules and mechanisms involved have been identified but how they are integrated and are able to commit a cell to apoptosis is poorly understood. For example, proapoptotic signals are sent through ER stress-induced upregulation of CHOP, also known as growth arrest and DNA damage-inducible gene 153 (GADD153; Oyadomari and Mori, 2004). CHOP is ubiquitously expressed at very low levels, but is robustly expressed by perturbations that induce stress in a wide variety of cells. CHOP expression in turn results in downregulation of the antiapoptotic protein Bcl-2, depletion of cellular glutathione and exaggerated production of reactive oxygen species (ROS; McCullough et al., 2001). Since both macrophages and SMCs transcriptionally upregulated CHOP upon treatment with spermine NONOate or thapsigargin to a similar extent, we do not believe that CHOP is responsible for selective induction of macrophage death. Moreover, CHOP−/− cells are still capable of undergoing ER stress-induced apoptosis, although with lower efficiencies (McCullough et al., 2001). However, CHOP was poorly expressed at the protein level in spermine NONOate-treated macrophages and highly expressed after thapsigargin treatment, a finding that correlated with the frequency of macrophage death (∼50 versus ∼90%, respectively). This apparent incongruity between CHOP protein levels and ER stress can be explained by a difference in the extent of ER stress induction. On the basis of CHOP mRNA levels, thapsigargin-induced ER stress was much more intense as compared to spermine NONOate-induced ER stress. On the other hand, it is noteworthy that translation of CHOP mRNA could be hampered in spermine NONOate-treated cells as ROS, including NO, induce oxidative RNA modifications and possibly RNA strand breaks (Martinet et al., 2004).
Translational attenuation occurs in an early phase of the ER stress response to reduce the load on the ER. This UPR event was also observed in the present study as de novo protein synthesis was significantly inhibited in macrophages and SMCs after treatment with spermine NONOate or thapsigargin. Recent evidence indicates that inhibition of protein synthesis drives selective induction of macrophage death in atherosclerotic plaques. Indeed, local administration of the protein synthesis inhibitor cycloheximide-induced macrophage apoptosis in plaques from cholesterol-fed rabbits without influencing the viability and reactivity of SMCs or the endothelium (Croons et al., 2007). Moreover, inhibition of translation by blocking activity of the protein characterized as mammalian target of rapamycin (mTOR) with the rapamycin-derivative everolimus led to a marked reduction in macrophage content without altering the amount of SMCs (Verheye et al., 2007), but in contrast to cycloheximide, autophagic cell death and not apoptosis was induced. Although the underlying mechanism of selective death is unknown, measurements of oxygen consumption (Bjornheden and Bondjers, 1987) as well as immunodetection of markers for DNA synthesis/repair (Lutgens et al., 1999; Martinet et al., 2002) indicate that plaque macrophages are metabolically highly active and thus more sensitive to protein synthesis inhibitors than the SMCs. In addition, inhibition of translation in SMCs induces a modulation towards a differentiated, quiescent, contractile phenotype (Martin et al., 2004), which may render SMCs relatively insensitive to cell death mediated by inhibition of protein translation. Therefore, besides differential expression of proapoptotic proteins such as CHOP, inhibition of translation may be responsible for selective ER stress-induced macrophage death by NO donors.
Taken together, NO-induced ER stress leads to selective depletion of macrophages in atherosclerotic plaques, probably via inhibition of protein synthesis. From a clinical perspective, it is important to note that protein synthesis inhibitors, in particular cycloheximide, cannot be administered systemically because they cause dramatic cell death in the liver (Higami et al., 2000). Local drug delivery by means of coated stents avoids unwanted systemic effects, but does not guarantee permanent depletion of macrophages due to the fast release rates (hours to months) of the coated drug. In case of short-term drug delivery, it is only a matter of time before blood monocytes reinvade the ‘purified' plaque. NO donors are widely used by patients with coronary artery disease to relieve the symptoms of ischaemia evoked by atherosclerosis and can be administered safely for many years (Herman and Moncada, 2005). We therefore believe that NO donors, if necessary in combination with a statin or local therapy (for example stent-based delivery of everolimus or cycloheximide), would offer new opportunities for a long-term macrophage depletory effect in atherosclerotic plaques.
Acknowledgments
This research was supported by the Fund for Scientific Research (FWO)-flanders (Belgium) (project nos. G.0308.04 and G.0113.06), the University of Antwerp (NOI-BOF) and the Bekales Foundation. We are indebted to Rita Van den Bossche, Hermine Fret, Lieve Svensson, Francis Terloo and Dominique De Rijck for their excellent technical assistance. Wim Martinet is a postdoctoral fellow of the FWO-Flanders.
Abbreviations
- agLDL
aggregated LDL
- AU
arbitrary unit
- BiP
immunoglobulin heavy-chain-binding protein
- CHOP
C/EBP homologous protein
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- Ire1
endoplasmic reticulum-to-nucleus signalling 1
- LDL
low-density lipoprotein
- PERK
PKR-like ER kinase
- SIN-1
3-morpholino-sydnonimine
- SMC
smooth muscle cell
- SNAP
S-nitroso-N-acetylpenicillamine
- UPR
unfolded protein response
- VCAM-1
vascular cell adhesion molecule 1
- XBP1
X-box-binding protein 1
Conflict of interest
The authors state no conflict of interest.
References
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Bjornheden T, Bondjers G. Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis. 1987;7:238–247. doi: 10.1161/01.atv.7.3.238. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- Croons V, Martinet W, Herman AG, Timmermans J-P, De Meyer GRY. Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J Pharmacol Exp Ther. 2007;320:986–993. doi: 10.1124/jpet.106.113944. [DOI] [PubMed] [Google Scholar]
- De Meyer GRY, Kockx MM, Knaapen MWM, Martinet W, De Cleen DMM, Bult H, et al. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability during cholesterol lowering in rabbits. J Cardiovasc Pharmacol. 2003;41:970–978. doi: 10.1097/00005344-200306000-00021. [DOI] [PubMed] [Google Scholar]
- DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M, Ostrowski J, Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14:S13–S22. [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Gutstein DE, Fuster V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res. 1999;41:323–333. doi: 10.1016/s0008-6363(98)00322-8. [DOI] [PubMed] [Google Scholar]
- Herman AG, Moncada S. Therapeutic potential of nitric oxide donors in the prevention and treatment of atherosclerosis. Eur Heart J. 2005;26:1945–1955. doi: 10.1093/eurheartj/ehi333. [DOI] [PubMed] [Google Scholar]
- Higami Y, Tanaka K, Tsuchiya T, Shimokawa I. Intravenous injection of cycloheximide induces apoptosis and up-regulates p53 and Fas receptor expression in the rat liver in vivo. Mutat Res. 2000;457:105–111. doi: 10.1016/s0027-5107(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Kim YC, Song SB, Lee MH, Kang KI, Lee H, Paik S-G, et al. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006;339:1007–1014. doi: 10.1016/j.bbrc.2005.11.099. [DOI] [PubMed] [Google Scholar]
- Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, et al. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:440–446. doi: 10.1161/01.ATV.0000057807.28754.7F. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- Liang S-L, Liu H, Zhou A. Lovastatin-induced apoptosis in macrophages through the Rac1/Cdc42/JNK pathway. J Immunol. 2006;177:651–656. doi: 10.4049/jimmunol.177.1.651. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- Lowik CW, Alblas MJ, van de Ruit M, Papapoulos SE, van der Pluijm G. Quantification of adherent and nonadherent cells cultured in 96-well plates using the supravital stain neutral red. Anal Biochem. 1993;213:426–433. doi: 10.1006/abio.1993.1442. [DOI] [PubMed] [Google Scholar]
- Lutgens E, de Muinck ED, Kitslaar PJEHM, Tordoir JHM, Wellens HJJ, Daemen MJAP. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Meyer GRY, Herman AG, Kockx MM. RNA damage in human atherosclerosis: pathophysiological significance and implications for gene expression studies. RNA Biol. 2004;1:128–131. doi: 10.4161/rna.2.1.1430. [DOI] [PubMed] [Google Scholar]
- Martinet W, Knaapen MWM, De Meyer GRY, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- Martinet W, Verheye S, De Meyer GRY. Selective depletion of macrophages in atherosclerotic plaques via macrophage-specific initiation of cell death. Trends Cardiovasc Med. 2007;17:69–75. doi: 10.1016/j.tcm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- McCarron RM, Goroff DK, Luhr JE, Murphy MA, Herscowitz HB. Methods for the collection of peritoneal and alveolar macrophages. Meth Enzymol. 1984;108:274–284. doi: 10.1016/s0076-6879(84)08092-7. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindala JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer UK, Ankarcrona M, Nicotera P, Brune B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–26. doi: 10.1016/0014-5793(94)01161-3. [DOI] [PubMed] [Google Scholar]
- Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Rabbani R, Topol EJ. Strategies to achieve coronary arterial plaque stabilization. Cardiovasc Res. 1999;41:402–417. doi: 10.1016/s0008-6363(98)00279-x. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Singh RJ, Hogg N, Joseph J, Konorev E, Kalyanaraman B. The peroxynitrite generator, SIN-1, becomes a nitric oxide donor in the presence of electron acceptors. Arch Biochem Biophys. 1999;361:331–339. doi: 10.1006/abbi.1998.1007. [DOI] [PubMed] [Google Scholar]
- Slager CJ, Wentzel JJ, Gijsen FJ, Thury A, van der Wal AC, Schaar JA, et al. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat Clin Pract Cardiovasc Med. 2005;2:456–464. doi: 10.1038/ncpcardio0298. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheye S, Martinet W, Kockx MM, Knaapen MWM, Salu K, Timmermans J-P, et al. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol. 2007;49:706–715. doi: 10.1016/j.jacc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]