Abstract
The gastrointestinal adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs) have been recognized since shortly after the introduction of aspirin to the marketplace over a century ago. However, the underlying pathogenesis of NSAID-induced gastropathy remains incompletely understood. Advances in understanding some of the factors that contribute to the mucosal injury have provided clues for the development of safer NSAIDs. The inhibitory effects of nitric oxide (NO) on NSAID-induced leukocyte adherence were exploited in the development of NO-releasing NSAIDs. As well as eliciting less gastrointestinal damage than conventional NSAIDs, these drugs do not elevate blood pressure and show anti-inflammatory effects, additional to those of the parent drugs. Modification of other drugs in a similar manner (i.e., NO-releasing derivatives) has similarly resulted in more effective drugs. More recently, hydrogen sulphide-releasing derivatives of NSAIDs and of other drugs, have been developed, based on the observed ability of H2S to reduce inflammation and pain in experimental models. H2S-releasing NSAIDs produce negligible gastric damage and exhibit enhanced anti-inflammatory potency as compared to the parent drugs. The NO-NSAIDs and H2S-releasing NSAIDs represent examples of new anti-inflammatory drugs with greatly reduced toxicity and improved therapeutic activity, both created through the concept of exploiting the beneficial effects of endogenous gaseous mediators.
Keywords: nonsteroidal anti-inflammatory drugs, inflammation, pain, nitric oxide, hydrogen sulphide, cyclooxygenase, prostaglandin, arthritis, inflammatory bowel disease
The clinical conundrum
Nonsteroidal anti-inflammatory drugs (NSAIDs) remain the first-line therapy for osteoarthritis and rheumatoid arthritis, despite many advances in the development of ‘disease-modifying' agents (such as anti-cytokine therapies) and despite a reasonably high incidence of significant adverse effects. The world market for NSAIDs exceeds £4 billion per year. The market expanded following the introduction of selective cyclooxygenase (COX)-2 inhibiting NSAIDs, but has also been steadily increasing because of ageing populations throughout the developed world (with the concomitant increase in prevalence of age-related diseases, such as arthritis). Use of the original NSAID, aspirin, has also been on the rise because of its perceived beneficial effects in attenuating the incidence of serious cardiovascular events (such as strokes and myocardial infarctions).
As the use of NSAIDs increases, so does the prevalence of NSAID-related adverse events. Most common among these are gastrointestinal (GI) bleeding and ulceration. The incidence of ‘clinically significant' GI adverse events with conventional NSAIDs has been estimated at between 2 and 4% (Steen et al., 2001). With selective COX-2 inhibitors, the results of large ‘outcomes studies' suggest that this rate may be reduced by ∼50–70% (Bombardier et al., 2000; Schnitzer et al., 2004), although the magnitude of the benefit continues to be debated (Juni et al., 2002; Hippisley-Cox et al., 2005).
Historical perspective
Aspirin entered the marketplace in 1898. It was developed as a better-tasting alternative to salicylate for the treatment of rheumatic conditions, but of course, it turned out to be distinct from salicylate in many ways. An association between its use and dyspepsia was quickly recognized, as it had been with the use of salicylate. However, direct evidence that aspirin caused bleeding in the stomach was not provided until 1938 (Douthwaite and Lintott, 1938).
In the decades that followed, numerous other studies confirmed that aspirin had the ability to induce damage in the stomach, and partly in response to this, dozens of new NSAIDs entered the marketplace. In general, these were more potent analgesic and anti-inflammatory agents than aspirin, but shared with aspirin the ability to promote ulceration and bleeding in the GI tract. The mechanism through which these agents elicited the mucosal injury, however, was not known. It was the discovery that NSAIDs inhibited prostaglandin synthesis (Vane, 1971) that provided an essential clue to the pathogenesis of NSAID-induced GI damage. The pioneering studies of Robert and colleagues in the early 1970s established the crucial importance of prostaglandins as mediators of mucosal defence against injury (Robert et al., 1976, 1979). Indeed, they provided the basis for the development of prostaglandin analogues as a prophylactic or curative therapy for NSAID-induced GI damage.
Prostaglandins are produced by the gastric mucosa and appear to mediate many of the components of what has been termed ‘gastric mucosal defence' (Wallace and Granger, 1996). This includes the maintenance of gastric blood flow during exposure to a noxious substance, secretion of bicarbonate and mucus by the surface epithelial cells, and the rapid repair of superficial injury through the process of epithelial restitution. Studies in the 1980s helped to define better the mechanism through which prostaglandins contributed to mucosal defence and the mechanisms through which NSAIDs impaired the ability of the GI mucosa to resist and respond to damage (reviewed by Wallace, 1997).
While suggestions of the existence of multiple forms of prostaglandin synthase, or COX, date back to 1972 (Flower and Vane, 1972), it was in 1991 that separate discoveries by two groups confirmed the existence of two distinct isoforms of COX (Kujubu et al., 1991; Xie et al., 1991). This triggered an enormous investment by pharmaceutical companies into the development of selective inhibitors of COX-2. This activity was based on the premise that the prostaglandins mediating inflammation were derived from this isoform, while the prostaglandins involved in protecting the GI tract were derived from COX-1. This has proven to be somewhat an over-simplification, although it is the case that COX-1 is the primary source of prostaglandin synthesis in the normal GI mucosa, and COX-2 appears to be the major source of prostaglandin synthesis at sites of inflammation. However, both COX-1 and COX-2 contribute significantly to GI mucosal defence, and both isoforms must be inhibited to generate mucosal injury in the absence of pre-existing injury (Wallace et al., 2000; Tanaka et al., 2001). Selective COX-2 inhibitors, such as rofecoxib and lumiracoxib, produce severe GI complications less frequently than conventional (non-selective) NSAIDs. The ‘residual' GI damage seen with these agents may be a consequence of the fact that COX-2 is rapidly expressed in response to GI injury (even when quite subtle), and contributes significantly to mucosal defence and repair in these circumstances (Davies et al., 1997b; Tanaka et al., 2002; Wallace and Devchand, 2005). Other adverse effects of selective COX-2 inhibitors, such as in the renal and cardiovascular systems, continue to be a significant limitation to their use (Mitchell and Warner, 2006; Zarraga and Schwarz, 2007). However, these adverse effects are unlikely to be produced only by selective COX-2 inhibitors. Indeed, there is good evidence that the same adverse effects are associated with the use of conventional NSAIDs (Kearney et al., 2006). Thus, although the development of selective COX-2 inhibitors has represented an important advance in addressing the GI toxicity of NSAIDs, it must be viewed only as an incremental advance given that GI and cardiovascular toxicity remain as important limitations to the use of these drugs.
The NO solution
In the 1970s and 1980s, the ‘gastroprotective' effects of prostaglandins were the focus of a great deal of research. In the late 1980s and early 1990s, nitric oxide (NO) emerged as another important mediator of mucosal defence. Application to the stomach of a solution of NO or of a NO donor significantly protected the mucosa from injury (MacNaughton et al., 1989; Kitagawa et al., 1990). However, it was another observation pertaining to the biology of NO that led to a novel approach to the development of GI-sparing NSAIDs.
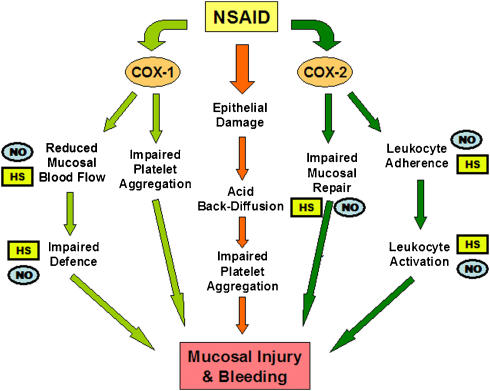
In the early 1990s, we provided evidence that NSAID-induced acute gastric mucosal damage was a neutrophil-dependent process. Rats that had been immuno-depleted of their circulating neutrophils developed very little gastric damage when given NSAIDs at doses that, in normal rats, caused widespread haemorrhagic lesions (Wallace et al., 1990). Moreover, interfering with the adherence of neutrophils to the vascular endothelium, through administration of monoclonal antibodies directed against leukocyte or endothelial adhesion molecules, also greatly reduced the severity of NSAID-induced gastric damage (Wallace et al., 1991, 1993). Various NSAIDs were found to trigger leukocyte adherence to the vascular endothelium, and this effect could be reversed by administration of prostaglandins (Asako et al., 1992a, 1992b). In the same period of time, NO was demonstrated to be an important modulator of adhesive interactions between leukocytes and the vascular endothelium (Kubes et al., 1991). This raised the possibility that protective effects of NO that had been observed in experimental models of gastric damage might be in part due to its ability to inhibit leukocyte–endothelial adhesion. Thus, we postulated that if NO could be delivered in small amounts (that is, not causing systemic hypotension) over a prolonged period of time, NSAID-induced leukocyte adherence should be prevented, as well as the usual reduction of gastric blood flow caused by NSAIDs and, thus, there should be a marked reduction in the severity of gastric mucosal injury (Figure 1). Testing this hypothesis was achieved through collaborative efforts that eventually led to the formation of NicOx S.A., a company with a mission to produce improved therapeutics by linking a NO-releasing moiety to existing anti-inflammatory drugs (an example is shown in Figure 2). We found that so-called ‘NO-NSAIDs' (also referred to as ‘COX-inhibiting NO donors') produced substantially less GI damage than the parent NSAIDs, despite markedly inhibiting gastric prostaglandin synthesis (Wallace et al., 1994a, 1994b). This profile was observed with many different types of NSAIDs, including aspirin (Wallace et al., 1995). The presence of the NO-releasing moiety was essential for the GI safety of these derivatives (Wallace et al., 2004). The NO-NSAIDs did not cause the decrease in gastric mucosal blood flow that was observed in rats following administration of the parent NSAID (Wallace et al., 1994a). As predicted, NO-NSAIDs did not cause the increase in leukocyte–endothelial adherence that occurred when conventional NSAIDs were administered to rats (Wallace et al., 1994b). Furthermore, unlike conventional NSAIDs, the NO-NSAIDs did not interfere with the healing of pre-existing gastric ulcers, and in some cases, accelerated experimental gastric ulcer healing (Elliott et al., 1995; Ma et al., 2002; Brzozowska et al., 2004). The reduced gastric injury that had been observed with NO-NSAIDs in experimental models was confirmed in human studies (Hawkey et al., 2003; Fiorucci et al., 2003b, 2003c). Moreover, the use of nitrovasodilators in combination with NSAIDs was shown to reduce significantly the incidence of GI bleeding associated with the latter (Lanas et al., 2000).
Figure 1.
Key steps in the pathogenesis of NSAID-induced gastric mucosal injury and bleeding. The steps where NO- and H2S-releasing drugs may exert beneficial effects are marked by NO (on blue) and HS (on yellow), respectively. COX, cyclooxygenase; NSAID, nonsteroidal anti-inflammatory drug.
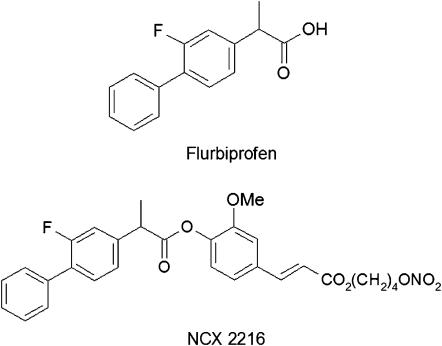
Figure 2.
Structures of flurbiprofen (conventional NSAID) and a NO-releasing derivative of flurbiprofen (NCX-2216). NSAID, nonsteroidal anti-inflammatory drug.
One of the surprising findings in studies of NO-NSAIDs was an increase in the anti-inflammatory and analgesic potency of these drugs (Davies et al., 1997a). Several mechanisms have been suggested as an explanation for these observations. First, NO itself can exhibit anti-inflammatory effects, such as inhibition of leukocyte adherence to the vascular endothelium (Kubes et al., 1991). NO has also been shown to exert analgesic actions (Ferreira, 1993) and to reduce the release of pro-inflammatory mediators from a number of cells, including mast cells (Hogaboam et al., 1993). Second, small amounts of NO have been shown to reduce expression of the inducible NO synthase (Cirino et al., 1996), which produces supra-physiological concentrations of NO that can contribute to inflammation and tissue injury. Third, NO can inhibit the activity of various caspases, thereby reducing synthesis of several pro-inflammatory cytokines (that is, interleukin (IL)-1, IL-18) and preventing apoptosis of endothelial cells (Fiorucci et al., 1999a, 1999b).
The positive results from studies with NO-releasing NSAIDs triggered the evaluation of NO-releasing derivatives of a number of other types of drugs, in an attempt to either reduce toxicity, boost potency, or both (Table 1). Thus, NO-releasing derivatives of glucocorticoids were shown to exhibit significantly less detrimental effects on bone (Paul-Clark et al., 2002), but to be more potent that the parent drugs in various inflammatory models (Paul-Clark et al., 2000, 2002; Fiorucci et al., 2002a; Turesin et al., 2003; Wallace et al., 2004). NO-releasing acetaminophen was shown to exhibit enhanced analgesic and anti-inflammatory effects, and to cause significantly less hepatic injury than the parent drug (Futter et al., 2001; Fiorucci et al., 2002a). NO-releasing derivatives of aspirin were found to be significantly more potent than aspirin in models of in vivo and in vitro models of colon cancer (Bak et al., 1998; Williams et al., 2001; Rigas and Williams, 2002). NO-aspirin also exhibited additional cardioprotective and antithrombotic effects, compared to those of aspirin (Wallace et al., 1995, 2002; Momi et al., 2000; Rossoni et al., 2000, 2001; Gresele et al., 2007). A NO-releasing derivative of mesalamine produced substantially greater beneficial effects in experimental colitis than the parent drug (Wallace et al., 1999).
Table 1.
Improvement of pharmacological profile of compounds through NO-releasing modification
| Drug class | Parent drugs | Added benefit | Key reviews and references |
|---|---|---|---|
| NSAID | Naproxen, diclofenac, flurbiprofen, ketoprofen, aspirin | ↓ Gastrointestinal toxicity ↑ Anti-inflammatory potency and/or efficacy ↓ Hypertension ↑ Efficacy and GI safety in experimental Alzheimer's disease ↑ Cardioprotective and antithrombotic ↑ Chemoprevention in colon cancer models |
Wallace et al. (2002); Wallace and Del Soldato (2003); Muscara et al. (2000); Jantzen et al. (2002); Gresele et al. (2007); Rigas and Williams (2002) |
| Glucocorticoid | Prednisolone, flunisolide, hydrocortisone | ↑ Anti-inflammatory potency/efficacy ↓ Adverse effects on bone |
Paul-Clark et al. (2000); Fiorucci et al. (2002b); Hyun et al. (2004); Wallace et al. (2004) |
| Analgesic | Paracetamol (acetaminophen) | ↑ Anti-inflammatory and analgesic potency/efficacy ↓ Adverse effects on liver |
Futter et al. (2001); Fiorucci et al. (2002a) |
| Gaba-pentin | ↑ Potency/efficacy | Wu et al. (2004) | |
| Mesalamine | 5-Aminosalicylic acid | ↑ Anti-inflammatory potency/efficacy | Wallace et al. (1999) |
| Vasodilator | Ursodeoxycholic acid | ↑ Potency/efficacy in portal hypertension | Fiorucci et al. (2003) |
| Statin | Pravastatin | ↑ Anti-thrombotic efficacy | Rossiello et al. (2005) |
It is the cardiovascular safety of NO-NSAIDs that is now seen as particularly attractive with respect to their prospects of gaining regulatory approval for the treatment of arthritis. This, of course, is in part a consequence of the increasing awareness of the cardiovascular toxicity associated with conventional and COX-2-selective NSAIDs. Animal studies demonstrated that a NO-releasing naproxen derivative did not exhibit the hypertensive effects of naproxen (Muscara et al., 1998, 2000, 2001). Phase III clinical trials of ‘naproxcinod', Nicox's lead drug, are currently underway, and aimed particularly at further demonstrating a safer cardiovascular profile of this drug as compared to older NSAIDs and selective COX-2 inhibitors. In studies completed thus far, the toxicity associated with NO-NSAIDs has mainly been attributable to the NSAID moiety (Lohmander et al., 2005), although in one study there were reports of orthostatic hypotension and dizziness experienced by some patients taking naproxcinod (likely to be related to the NO release from this compound) (Michael Hill et al., 2006).
SHH—another solution
As was the case for NO before the 1980s, hydrogen sulphide (H2S) has until recently been better known as an industrial pollutant than as an endogenous mediator of numerous physiological processes (Wang, 2002; Fiorucci et al., 2006). Nevertheless, the notion that H2S may have beneficial effects is not new. For centuries, bathing in H2S-rich mineral springs has been perceived to relieve pain and reduce inflammation, modulate the immune system and improve blood flow. In recent years, scientific evidence to support many of these contentions has been generated. H2S is produced through a number of pathways, the most common being related to the metabolism of L-cysteine, cystine and homocysteine (Wang, 2002). Endogenous levels of H2S have been reported to be as high as 160 μM in the brain, with serum levels of 30–100 μM (Wang, 2002). Even after administration of H2S donors in doses that produce pharmacological effects, the concentration of H2S in plasma seldom exceeded the normal range, or did so for a very brief period of time (Li et al., 2005; Distrutti et al., 2006b), because of the highly efficient systems for metabolizing, scavenging and sequestering H2S (Wang, 2002; Fiorucci et al., 2006). Toxic effects of H2S are observed in the lung with concentrations in excess of 250 μM, and coma and death can occur with concentrations above 1000 μM (Milby and Baselt, 1999).
In various models of tissue injury, production of H2S is markedly enhanced (Bhatia et al., 2005; Yusuf et al., 2005). Whether H2S is primarily detrimental or beneficial in such circumstances is not yet clear. As is the case with NO, physiological concentrations of H2S are likely to be beneficial, while concentrations many times greater than the physiological range would tend to be detrimental. The highest levels of H2S in the body are found in the lumen of the colon (low millimolar concentrations) (Magee et al., 2000), but in this case the primary source of this gaseous mediator is the commensal flora. Recent studies suggest that H2S is an inorganic substrate for mammalian mitochondria, and the mitochondria of colonic epithelial cells may be particularly well adapted for the use of the substrate (Goubern et al., 2007).
H2S can relax vascular smooth muscle, possibly via activation of ATP-sensitive K+ channels (K+ATP) (Wang, 2002; Tang et al., 2005). Recently, potent anti-inflammatory effects of H2S were demonstrated. For example, H2S donors suppressed leukocyte adherence to the vascular endothelium and reduced leukocyte extravasation and oedema formation (Zanardo et al., 2006). Inhibition of endogenous H2S synthesis, on the other hand, triggered leukocyte adherence to the vascular endothelium and enhanced oedema formation (Zanardo et al., 2006). These actions of H2S also appeared to be mediated via activation of K+ATP channels. H2S has been shown to induce apoptosis of neutrophils (Mariggio et al., 1998), which could contribute to resolution of inflammation (Gilroy et al., 2004). H2S has also been reported to inhibit oxidative damage to tissue, in part through scavenging of peroxynitrite (Whiteman et al., 2004, 2005). H2S donors have been shown to decrease endotoxin-induced cytokine expression and NO production (Hu et al., 2007; Li et al., 2007). H2S has also been shown to reduce visceral pain perception, which may also be mediated via activation of K+ATP channels (Distrutti et al., 2006a). Indeed, analgesic effects of NSAIDs have been suggested to be mediated, at least in part, via K+ATP channels (Ortiz et al., 2001).
H2S is also produced by the gastric mucosa, and like NO, contributes to the ability of this tissue to resist damage induced by luminal substances (Fiorucci et al., 2005). Surprisingly, H2S synthesis was found to be significantly reduced following administration of NSAIDs, apparently through suppression of the expression of cystathionine-γ-lyase, one of the key enzymes for conversion of L-cysteine into H2S (Fiorucci et al., 2005). Thus, suppression of mucosal H2S synthesis may represent another mechanism, aside from suppression of COX activity, through which NSAIDs produce GI damage. Administration of H2S donors could prevent the decrease in gastric blood flow induced by NSAIDs, as well as diminishing NSAID-induced leukocyte adherence. It also decreased the NSAID-induced accumulation of leukocytes in the gastric mucosa, as well as expression of endothelial and leukocyte adhesion molecules (Fiorucci et al., 2005; Zanardo et al., 2006; Wallace et al., 2007). Several of these H2S-induced effects were inhibited by glibenclamide and/or mimicked by pinacidil, suggesting that they were produced via activation of K+ATP channels.
Building on these observations, we explored the effects of several H2S-releasing derivatives of NSAIDs (synthesized by Antibe Therapeutics, Toronto, Canada), to determine if release of small amounts of this mediator could counteract the detrimental effects of the NSAID moiety. Indeed, it appeared from the studies described above that H2S had the potential to interfere with many of the key events in the pathogenesis of NSAID-induced mucosal injury in similar fashion to NO (Figure 1). As was the case with NO-NSAIDs, the H2S-releasing NSAIDs were substantially better tolerated, in terms of gastric damage, than the parent drugs. At single doses of more than five times the ED50 for anti-inflammatory activity in the carrageenan paw oedema model in rats, no gastric damage was detected with an H2S-releasing derivative of diclofenac (Figure 3) (Wallace et al., 2007). With administration of this same dose of the compound three times over 24 h, very low levels of intestinal damage were observed, at least 90% less than that observed in rats given diclofenac at an equimolar dose (Wallace et al., 2007). Moreover, there was no change in haematocrit in the rats treated with the H2S-releasing derivative, while diclofenac administration resulted in a decrease in haematocrit of 50%, consistent with the widespread bleeding that was evident in the intestine (Wallace et al., 2007).
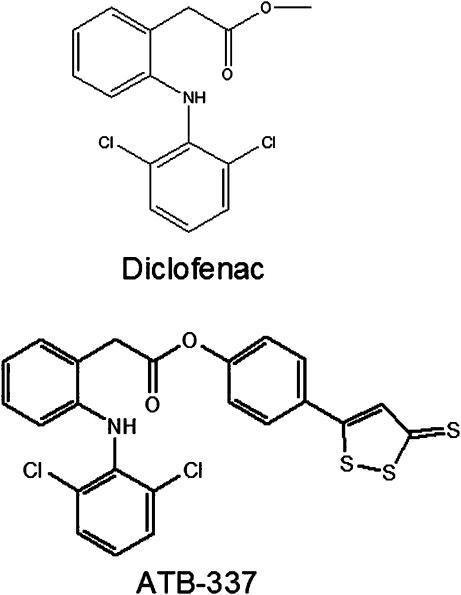
Figure 3.
Structures of diclofenac (conventional NSAID) and a H2S-releasing derivative of diclofenac (ATB-337). NSAID, nonsteroidal anti-inflammatory drug.
The benefit provided by the H2S-releasing moiety was not only in terms of reduced GI toxicity. We also observed a 2- to 3-fold increase in anti-inflammatory potency of the H2S-diclofenac compound as compared to diclofenac (Wallace et al., 2007). This increase in potency has also been observed with other H2S-releasing compounds that we and others have evaluated (Fiorucci et al., 2007; Li et al., 2007). The increased anti-inflammatory potency may be related to more potent suppression of COX activity. Alternatively, suppression of pro-inflammatory cytokine synthesis by H2S-releasing NSAIDs may explain their increased anti-inflammatory activity (Fiorucci et al., 2005; Li et al., 2007).
The anti-inflammatory and visceral analgesic effects of H2S have also been exploited recently in the design of novel derivatives of mesalamine for the treatment of inflammatory bowel disease (IBD). Mesalamine is the first-line therapy for IBD, but it is a weak drug, so doses of up to 6 g/day are necessary. Moreover, mesalamine is only effective in mild-to-moderate cases of IBD. A mesalamine derivative that includes an H2S-releasing moiety (ATB-429) was recently shown to exhibit markedly enhanced anti-inflammatory actions in experimental colitis (Fiorucci et al., 2007). The H2S-releasing moiety alone did not produce significant beneficial effects in this model of colitis (Fiorucci et al., 2007). Unlike mesalamine, the H2S-releasing derivative significantly suppressed the expression of several pro-inflammatory cytokines in the colon, as well as reducing granulocyte levels in colonic tissue to those of healthy controls (Fiorucci et al., 2007). Moreover, the drug exhibited significant analgesic effects in a rat model of visceral pain (Distrutti et al., 2006b). This may have particular clinical significance given that abdominal pain is one of the most common and debilitating symptoms of IBD.
On the horizon
The great challenge for those attempting to develop safer NSAIDs is shifting from a focus on the GI toxicity to the increasingly more appreciated cardiovascular toxicity. While selective COX-2 inhibitors represented an important advance in anti-inflammatory therapy, there is plenty of room for improvement. NO-NSAIDs and several other NO-releasing drugs (Table 1) are in advanced clinical trials, so in the near future the degree to which these drugs represent significant advances in a clinical setting will become clearer. While the development of H2S-releasing anti-inflammatory drugs is in its infancy, the preclinical data available thus far provide cause for optimism.
Acknowledgments
The work reviewed in this paper was supported by grants from the Canadian Institutes of Health Research. The author is an Alberta Heritage Foundation for Medical Research Scientist, and holds a Canada Research Chair in Inflammation.
Abbreviations
- COX
cyclooxygenase
- GI
gastrointestinal
- NSAID
nonsteroidal anti-inflammatory drug
Conflict of interest
The author holds shares in Antibe Therapeutics Inc., a company focused on hydrogen sulphide-releasing drugs.
References
- Asako H, Kubes P, Wallace JL, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992a;262:G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- Asako H, Kubes P, Wallace JL, Wolf RE, Granger DN. Modulation of leukocyte adhesion to rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992b;103:146–152. doi: 10.1016/0016-5085(92)91107-f. [DOI] [PubMed] [Google Scholar]
- Bak AW, McKnight W, Li P, Del Soldato P, Calignano A, Cirino G, et al. Cyclooxygenase-independent chemoprevention with an aspirin derivative in a rat model of colonic adenocarcinoma. Life Sci. 1998;62:PL367–PL373. doi: 10.1016/s0024-3205(98)00191-x. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulphide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- Brzozowska I, Targosz A, Sliwowski Z, Kwiecien S, Drozdowicz D, Pajdo R, et al. Healing of chronic gastric ulcers in diabetic rats treated with native aspirin, nitric oxide (NO)-derivative of aspirin and cyclooxygenase (COX)-2 inhibitor. J Physiol Pharmacol. 2004;55:773–790. [PubMed] [Google Scholar]
- Cirino G, Wheeler-Jones CP, Wallace JL, Del Soldato P, Baydoun AR. Inhibition of inducible nitric oxide synthase expression by novel nonsteroidal anti-inflammatory derivatives with gastrointestinal-sparing properties. Br J Pharmacol. 1996;117:1421–1426. doi: 10.1111/j.1476-5381.1996.tb15301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM, Roseth AG, Appleyard CB, McKnight W, Del Soldato P, Calignano A, et al. NO-naproxen vs naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment Pharmacol Ther. 1997b;11:69–79. doi: 10.1046/j.1365-2036.1997.115286000.x. [DOI] [PubMed] [Google Scholar]
- Davies NM, Sharkey KA, Asfaha S, MacNaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther. 1997a;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulphide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006a;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1, 2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulphide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006b;319:447–458. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- Douthwaite AH, Lintott SAM. Gastroscopic observation of the effect of aspirin and certain other substances on the stomach. Lancet. 1938;2:1222–1225. [Google Scholar]
- Elliott SN, McKnight W, Cirino G, Wallace JL. A nitric oxide-releasing nonsteroidal anti-inflammatory drug accelerates gastric ulcer healing in rats. Gastroenterology. 1995;109:524–530. doi: 10.1016/0016-5085(95)90341-0. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. The role of interleukins and nitric oxide in the mediation of inflammatory pain and its control by peripheral analgesics. Drugs. 1993;46 Suppl 1:1–9. doi: 10.2165/00003495-199300461-00003. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Brancaleone V, Sanpaolo L, Orlandi S, Distrutti E, et al. NCX-1000, a nitric oxide-releasing derivative of ursodeoxycholic acid, ameliorates portal hypertension and lowers norepinephrine-induced intrahepatic resistance in the isolated and perfused rat liver. J Hepatol. 2003;39:932–939. doi: 10.1016/s0168-8278(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Del Soldato P, Flower RJ, Clark MJ, et al. NCX-1015, a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA. 2002a;99:15770–15775. doi: 10.1073/pnas.232583599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulphide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Mencarelli A, Palazzetti B, Alvarez-Miller L, Muscara M, et al. A NO-releasing derivative of acetaminophen spares the liver by acting at several checkpoints in the Fas pathway. Br J Pharmacol. 2002b;135:589–599. doi: 10.1038/sj.bjp.0704500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, et al. Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999a;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulphide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Federici B, Antonelli E, Distrutti E, Morelli O, et al. Nitric oxide-releasing NSAIDs inhibit interleukin-1β converting enzyme-like cysteine proteases and protect endothelial cells from apoptosis induced by TNFα. Aliment Pharmacol Ther. 1999b;13:421–435. doi: 10.1046/j.1365-2036.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Gresele P, Faccino RM, del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003b;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, et al. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc Natl Acad Sci USA. 2003c;100:10937–10941. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol) Nature. 1972;240:410–411. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- Futter LE, al-Swayeh OA, Moore PK. A comparison of the effect of nitroparacetamol and paracetamol on liver injury. Br J Pharmacol. 2001;132:10–12. doi: 10.1038/sj.bjp.0703837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillard F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- Gresele P, Migliacci R, Procacci A, De Monte P, Bonizzoni E. Prevention by NCX 4016, a nitric oxide-donating aspirin, but not by aspirin, of the acute endothelial dysfunction induced by exercise in patients with intermittent claudication. Thromb Haemost. 2007;97:444–450. [PubMed] [Google Scholar]
- Hawkey C, Jones JI, Atherton CT, Skelly MM, Bebb JR, Fagerholm U, et al. Gastrointestinal safety of AZD3582, a cyclooxygenase inhibiting nitric oxide donator: proof of concept study in humans. Gut. 2003;52:1537–1542. doi: 10.1136/gut.52.11.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional nonsteroidal anti-inflammatory drugs: population based nested case–control analysis. BMJ. 2005;331:1310–1316. doi: 10.1136/bmj.331.7528.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Befus AD, Wallace JL. Modulation of rat mast cell reactivity by IL-β. Divergent effects on nitric oxide and platelet-activating factor release. J Immunol. 1993;151:3767–3774. [PubMed] [Google Scholar]
- Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulphide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- Hyun E, Bolla M, Steinhoff M, Wallace JL, Soldato PD, Vergnolle N. Anti-inflammatory effects of nitric oxide-releasing hydrocortisone NCX 1022, in a murine model of contact dermatitis. Br J Pharmacol. 2004;143:618–625. doi: 10.1038/sj.bjp.0705854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, et al. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüni P, Rutjes JP, Dieppe PA. Are selective COX 2 inhibitors superior to traditional non-steroidal anti-inflammatory drugs. BMJ. 2002;324:1287–1288. doi: 10.1136/bmj.324.7349.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Takeda F, Kohei H. Effect of endothelium-derived relaxing factor on the gastric lesion induced by HCl in rats. J Pharmacol Exp Ther. 1990;253:1133–1137. [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- Lanas A, Bajador E, Serrano P, Fuentes J, Carreno S, Guardia J, et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal anti-inflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834–839. doi: 10.1056/NEJM200009213431202. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulphide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, McKeith D, Svensson O, Malmenäs M, Bolin L, Kalla A, et al. A randomised, placebo controlled, comparative trial of the gastrointestinal safety and efficacy of AZD3582 versus naproxen in osteoarthritis. Ann Rheum Dis. 2005;64:449–456. doi: 10.1136/ard.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Del Soldato P, Wallace JL. Divergent effects of new cyclooxygenase inhibitors on gastric ulcer healing: shifting the angiogenic balance. Proc Natl Acad Sci USA. 2002;99:13243–13247. doi: 10.1073/pnas.202392199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton WK, Cirino G, Wallace JL. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45:1869–1876. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000;72:1488–4894. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- Mariggio MA, Minunno V, Riccardi S, Santacroce R, De Rinaldis P, Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- Michael Hill C, Sindet-Pederson S, Seymour RA, Hawkesford JE, Coulthard P, Lamey PJ, et al. Analgesic efficacy of the cyclooxygenase-inhibiting nitric oxide donor AZD3582 in postoperative dental pain: Comparison with naproxen and rofecoxib in two randomized, double-blind, placebo-controlled studies. Clin Ther. 2006;28:1279–1295. doi: 10.1016/j.clinthera.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Milby TH, Baselt RC. Hydrogen sulfide poisoning: clarification of some controversial issues. Am J Ind Med. 1999;35:192–195. doi: 10.1002/(sici)1097-0274(199902)35:2<192::aid-ajim11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- Momi S, Emerson M, Paul W, Leone M, Mezzasoma AM, Del Soldato P, et al. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur J Pharmacol. 2000;397:177–185. doi: 10.1016/s0014-2999(00)00223-5. [DOI] [PubMed] [Google Scholar]
- Muscará MN, Lovren F, McKnight W, Dicay M, Del Soldato P, Triggle CR, et al. Vasorelaxant effects of a nitric oxide-releasing aspirin derivative in normotensive and hypertensive rats. Br J Pharmacol. 2001;133:1314–1322. doi: 10.1038/sj.bjp.0704209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscará MN, Lovren F, McKnight W, Triggle CR, Cirino G, Wallace JL. Anti-hypertensive properties of a nitric oxide-releasing naproxen derivative in 2-kidney, 1-clip rats. Am J Physiol. 2000;279:H528–H535. doi: 10.1152/ajpheart.2000.279.2.H528. [DOI] [PubMed] [Google Scholar]
- Muscará MN, McKnight W, Wallace JL. Effect of a nitric oxide-releasing naproxen derivative on hypertension and gastric damage induced by chronic nitric oxide inhibition in the rat. Life Sci. 1998;62:PL235–PL240. doi: 10.1016/s0024-3205(98)00072-1. [DOI] [PubMed] [Google Scholar]
- Ortiz MI, Castaneda-Hernandez G, Rosas R, Vidal-Cantu GC, Granados-Soto V. Evidence for a new mechanism of action of diclofenac: activation of K+ channels. Proc West Pharmacol Soc. 2001;44:19–21. [PubMed] [Google Scholar]
- Paul-Clark M, Del Soldato P, Fiorucci S, Flower RJ, Perretti M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti-inflammatory properties. Br J Pharmacol. 2000;131:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Clark MJ, Mancini L, Del Soldato P, Flower RJ, Perretti M. Potent antiarthritic properties of a glucocorticoid derivative, NCX-1015, in an experimental model of arthritis. Proc Natl Acad Sci USA. 2002;99:1677–1682. doi: 10.1073/pnas.022641099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B, Williams JL. NO-releasing NSAIDs and colon cancer chemoprevention: A promising novel approach. Int J Oncol. 2002;20:885–990. [PubMed] [Google Scholar]
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- Robert A, Schultz JR, Nezamis JE, Lancaster C. Gastric antisecretory and antiulcer properties of PGE2, 15-methyl PGE2, and 16, 16-dimethyl PGE2. Intravenous, oral and intrajejunal administration. Gastroenterology. 1976;70:359–370. [PubMed] [Google Scholar]
- Rossiello MR, Momi S, Caracchini R, Giannini S, Guglielmini G, Monopoli A, et al. A novel nitric oxide-releasing statin derivative exerts an antiplatelet/antithrombotic activity and inhibits tissue factor expression. J Thromb Haemost. 2005;3:2554–2562. doi: 10.1111/j.1538-7836.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Berti M, De Gennaro Colonna V, Del Soldato P, Berti F. myocardial protection by the nitroderivative of aspirin, NCX 4016: in vitro and in vivo experiments in the rabbit. Ital Heart J. 2000;1:146–155. [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, de Gennaro Colonna V, Bernareggi M, Berti F. The nitroderivative of aspirin, NCX 4016, reduces infarct size caused by myocardial ischemia-reperfusion in the anesthetized rat. J Pharmacol Exp Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- Steen KS, Lems WF, Aertsen J, Bezemer D, Dijkmans BA. Incidence of clinically manifest ulcers and their complications in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:443–447. doi: 10.1136/ard.60.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Araki H, Komoike Y, Hase S, Takeuchi K. Inhibition of both COX-1 and COX-2 is required for development of gastric damage in response to nonsteroidal sulphide anti-inflammatory drugs. J Physiol Paris. 2001;95:21–27. doi: 10.1016/s0928-4257(01)00005-5. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Hase S, Miyazawa T, Takeuchi K. Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: a key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J Pharmacol Exp Ther. 2002;300:754–761. doi: 10.1124/jpet.300.3.754. [DOI] [PubMed] [Google Scholar]
- Tang G, Wu L, Liang W, Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulphide in vascular smooth muscle cells. Mol Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- Turesin F, del Soldato P, Wallace JL. Enhanced anti-inflammatory potency of a nitric oxide-releasing prednisolone derivative in the rat. Br J Pharmacol. 2003;139:966–972. doi: 10.1038/sj.bjp.0705324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Del Soldato P. The therapeutic potential of NO-NSAIDs. Fundam Clin Pharmacol. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Devchand PR. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defence. Br J Pharmacol. 2005;145:275–282. doi: 10.1038/sj.bjp.0706201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Arfors K-E, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991;100:878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulphide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of nitric oxide-releasing aspirin. Nature Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Del Soldato P, Baydoun AR, Cirino G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J Clin Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, et al. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993;265:G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Muscara MN, de Nucci G, Zamuner S, Cirino G, del Soldato P, et al. Gastric tolerability and prolonged prostaglandin inhibition in the brain with a nitric oxide-releasing flurbiprofen derivative, NCX-2216 [3-[4-(2-fluoro-α-methyl-[1,1′-biphenyl]-4-acetyloxy)-3-methoxyphenyl]-2-propenoic acid 4-nitrooxy butyl ester] J Pharmacol Exp Ther. 2004;309:626–633. doi: 10.1124/jpet.103.063453. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Reuter B, Cicala C, McKnight W, Grisham M, Cirino G. A diclofenac derivative without ulcerogenic properties. Eur J Pharmacol. 1994a;257:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Reuter B, Cicala C, McKnight W, Grisham MB, Cirino G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994b;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Rizzo G, Cirino G, Del Soldato P, Fiorucci S. Enhanced anti-inflammatory potency of a nitric oxide-releasing derivative of flunisolide: role of nuclear factor-κB. J Pharmacol Exp Ther. 2004;310:1096–1102. doi: 10.1124/jpet.104.067850. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vergnolle N, Muscara MN, Asfaha S, Chapman K, McKnight W, et al. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology. 1999;117:557–566. doi: 10.1016/s0016-5085(99)70448-8. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Siau JL, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger'. J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain. Biochem Biophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- Williams JL, Borgo S, Hasan I, Castillo E, Traganos F, Rigas B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer chemoprevention. Cancer Res. 2001;61:3285–3289. [PubMed] [Google Scholar]
- Wu WP, Hao JX, Ongini E, Impagnatiello F, Presotto C, Wiesenfeld-Hallin Z, et al. A nitric oxide (NO)-releasing derivative of gabapentin, NCX 8001, alleviates neuropathic pain-like sulphide after spinal cord and peripheral nerve injury. Br J Pharmacol. 2004;141:65–74. doi: 10.1038/sj.bjp.0705596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulphide biosynthesis. Biochem Biophys Res Commun. 2005;333:1146–1152. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulphide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zarraga IG, Schwarz ER. Coxibs and heart disease: what we have learned and what else we need to know. J Am Coll Cardiol. 2007;49:1–14. doi: 10.1016/j.jacc.2006.10.003. [DOI] [PubMed] [Google Scholar]