Abstract
Background and purpose:
Licofelone is a dual inhibitor of the cyclooxygenase and 5-lipoxygenase (5-LO) pathway, and has been developed for the treatment of inflammatory diseases. Here, we investigated the molecular mechanisms underlying the inhibition by licofelone of the formation of 5-LO products.
Experimental approach:
The efficacy of licofelone to inhibit the formation of 5-LO products was analysed in human isolated polymorphonuclear leukocytes (PMNL) or transfected HeLa cells, as well as in cell-free assays using respective cell homogenates or purified recombinant 5-LO. Moreover, the effects of licofelone on the subcellular redistribution of 5-LO were studied.
Key results:
Licofelone potently blocked synthesis of 5-LO products in Ca2+-ionophore-activated PMNL (IC50=1.7 μM) but was a weak inhibitor of 5-LO activity in cell-free assays (IC50≫10 μ M). The structures of licofelone and MK-886, an inhibitor of the 5-LO-activating protein (FLAP), were superimposable. The potencies of both licofelone and MK-886 in ionophore-activated PMNL were impaired upon increasing the concentration of arachidonic acid, or under conditions where 5-LO product formation was evoked by genotoxic, oxidative or hyperosmotic stress. Furthermore, licofelone prevented nuclear redistribution of 5-LO in ionophore-activated PMNL, as had been observed for FLAP inhibitors. Finally, licofelone as well as MK-886 caused only moderate inhibition of the synthesis of 5-LO products in HeLa cells, unless FLAP was co-transfected.
Conclusions and implications:
Our data suggest that the potent inhibition of the biosynthesis of 5-LO products by licofelone requires an intact cellular environment and appears to be due to interference with FLAP.
Keywords: 5-lipoxygenase, 5-lipoxygenase-activating protein, leukotriene, inflammation, arachidonic acid, polymorphonuclear leukocytes
Introduction
Metabolism of free arachidonic acid (AA), liberated by phospholipase A2 enzymes, leads to the formation of prostanoids and leukotrienes (LTs) that are considered as potent mediators of inflammatory reactions (for review, see Funk, 2001). Whereas the cyclooxygenases (COXs)-1 and -2 catalyse the initial steps in the biosynthesis of prostanoids, 5-lipoxygenase (5-LO) is the key enzyme in the generation of LTs. According to the pivotal and complex functions of these eicosanoids in the pathophysiology of inflammation and also in normal physiology, a well-balanced pharmacological intervention with the respective enzymes and receptors involved is required for efficient and safe clinical therapy. Inhibitors of COX enzymes provide substantial benefit in the treatment of fever, pain and inflammatory diseases (that is, arthritis), whereas inhibitors of the 5-LO pathway have proven beneficial mainly in the therapy of asthma (Funk, 2001).
Pharmacological strategies for repression of LT synthesis encompass direct inhibition of the 5-LO enzyme using lipophilic redox-active compounds, iron ligands and nonredox-type 5-LO inhibitors, as well as the blockade of the 5-LO-activating protein (FLAP) (for review see Werz, 2002). FLAP, a membrane-bound 18 kDa protein, was discovered as a MK-886-binding protein, being necessary for cellular LT biosynthesis in vitro and in vivo. Thus, MK-886 strongly inhibited 5-LO activity in intact activated leukocytes and blocked translocation of 5-LO, but failed to suppress potently 5-LO activity in cell-free systems (Gillard et al., 1989; Dixon et al., 1990; Miller et al., 1990; Rouzer et al., 1990). Cells that lack FLAP produce low amounts of LTs, in spite of overexpressing 5-LO (Dixon et al., 1990). Mice, deficient in FLAP fail to produce LTs and exhibit reduced symptoms of experimental inflammation, comparable to mice lacking 5-LO (Chen et al., 1994; Byrum et al., 1997). Mechanistically, FLAP may function as a substrate transfer protein, facilitating the access of 5-LO towards AA (Abramovitz et al., 1993; Mancini et al., 1993).
Although many inhibitors selective for either the COX or the 5-LO pathway are currently available, recent developments indicate a greater efficacy of dual COX/5-LO inhibitors associated also with higher gastrointestinal safety over inhibitors with activity towards a single enzyme (Celotti and Laufer, 2001). Licofelone was developed as a dual COX/5-LO inhibitor (Ding and Cicuttini, 2003), based on its ability to suppress COX1/2 and 5-LO activities in intact cell assays (Laufer et al., 1994a, 1994b), and is currently undergoing Phase III clinical trials for therapy of osteoarthritis. Depending on the assay system, the half-maximal inhibitory concentration (IC50) values for 5-LO product synthesis induced by stimulation with ionophore A23187 have been shown to be in the range of 0.21–3.8 μM (Laufer et al., 1994b; Rotondo et al., 2002; Tries et al., 2002).
Despite the well-documented investigations on the efficacy of licofelone in different intact cell assays, no data regarding the inhibition of 5-LO activity in cell-free systems (that is, crude 5-LO in homogenates or purified enzyme) are yet available. In this study, we attempted to explore the molecular mechanism underlying the potent inhibition of cellular LT synthesis by licofelone. We found that with respect to interference with 5-LO product formation, licofelone shares several properties with the FLAP inhibitor MK-886. Thus, licofelone potently inhibits 5-LO activity and nuclear 5-LO translocation in intact cells but exerts only weak 5-LO inhibitory actions in cell-free assays, and we suggest that licofelone may act primarily on FLAP.
Materials and methods
Cell culture and transient transfections
Human polymorphonuclear leukocytes (PMNL) were freshly isolated from leukocyte concentrates obtained at St Markus Hospital (Frankfurt, Germany). In brief, venous blood was taken from healthy adult donors and centrifuged at 4000 g for 20 min at 20°C for preparation of leukocyte concentrates. PMNL were promptly isolated by dextran sedimentation, centrifugation on Nycoprep cushions (PAA Laboratories, Linz, Austria), and hypotonic lysis of erythrocytes as described previously (Werz et al., 2002a). PMNL (7.5 × 106 cells ml−1; purity >96–97%) were finally re-suspended in phosphate-buffered saline, pH 7.4 (PBS) plus 1 mg ml−1 glucose (PG buffer), or alternatively in PBS containing 1 mg ml−1 glucose and 1 mM CaCl2 (PGC buffer) as indicated.
The coding sequence of FLAP was amplified by PCR from cDNA of Mono Mac 6 cells using Pfu polymerase (Promega, Mannheim, Germany). Restriction sites for endonucleases were introduced by the PCR primers 5′-GGTGGAATTCATGG ATCAAGAAACTGTAG (EcoRI, forward) and 5′-GGCGACGAG ATCTACCAACCCCATATTCAGCAGAG (BglII, reverse). After amplification, the PCR product was digested with the respective enzymes (Promega) and cloned into the eukaryotic expression vector pSG5 (Stratagene, Amsterdam, The Netherlands) using T4-DNA ligase (New England Biolabs, Beverly, MA, USA). Restriction digestion identified positive clones expressing pSG5-FLAP, which were further proven by sequencing. HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum and 100 μg ml−1 streptomycin and 100 U ml−1 penicillin at 37°C in a 5% CO2 incubator. Plasmid DNA (pcDNA3.1–5LO (Provost et al., 2001), pSG5 (empty vector) and pSG5-FLAP, 10 μg each) was transiently transfected into HeLa cells using the calcium–phosphate method, cultured for 48 h, and assayed for 5-LO product formation as described elsewhere (Fischer et al., 2003).
Expression and purification of 5-LO from Escherichia coli
Expression of 5-LO was performed in E. coli JM 109 cells, transfected with pT3–5LO, and purification of 5-LO was performed as described previously (Fischer et al., 2003). In brief, E. coli were harvested and lysed by incubation in 50 mM triethanolamine/HCl, pH 8.0, 5 mM EDTA, soybean trypsin inhibitor (60 μg ml−1), 1 mM phenylmethylsulphonyl fluoride (PMSF) and lysozyme (500 μg ml−1) homogenized by sonication (3 × 15 s) and centrifuged at 19 000 g for 15 min. Proteins including 5-LO were precipitated with 50% saturated ammonium sulphate during stirring on ice for 60 min. The precipitate was collected by centrifugation at 16 000 g for 25 min and the pellet was re-suspended in 20 ml PBS containing 1 mM EDTA and 1 mM PMSF. After centrifugation at 100 000 g for 70 min at 4°C, the 100 000 g supernatant was applied to an ATP-agarose column (Sigma A2767; Deisenhofen, Germany), and the column was eluted as described previously (Brungs et al., 1995). Partially purified 5-LO was immediately used for in vitro activity assays.
Determination of 5-LO product formation in intact cells
For assays of intact cells, 7.5 × 106 freshly isolated PMNL or 2 × 106 HeLa cells were finally re-suspended in 1 ml PGC buffer. After preincubation with the indicated compounds at 37°C, 5-LO product formation was started by addition of the indicated stimuli plus exogenous AA as indicated. After 10 min at 37°C, the reaction was stopped with 1 ml of methanol and 30 μl of 1 N HCl, 200 ng prostaglandin (PG) B1 and 500 μl of PBS were added. Formed 5-LO metabolites were extracted and analysed by HPLC as described (Werz and Steinhilber, 1996). 5-LO product formation is expressed as nanograms of 5-LO products per 106 cells, which includes LTB4 and its all-trans isomers, 5(S),12(S)-di-hydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid (5(S),12(S)-DiHETE), and 5(S)-hydro(pero)xy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-H(p)ETE). Cysteinyl LTs (LTC4, D4 and E4) were not detected and oxidation products of LTB4 were not determined.
Determination of 5-LO product formation in cell-free systems
For determination of 5-LO activity in cell homogenates, 7.5 × 106 freshly isolated PMNL were re-suspended in PBS containing 1 mM EDTA, sonicated (3 × 10 s) at 4°C, and 1 mM ATP was added. For determination of the activity of purified human recombinant 5-LO, partially purified 5-LO (0.5 μg in 5 μl) was added to 1 ml of a 5-LO reaction mix (PBS, pH 7.4, 1 mM EDTA, 25 μg ml−1 phosphatidylcholine, 1 mM ATP, and 20 μg ml−1 γ-globulin). Samples of either cell homogenates or partially purified 5-LO were supplemented with dithiothreitol (DTT) (1 mM) and licofelone or MK-886 as indicated. After 5–10 min at 4°C, samples were prewarmed for 30 s at 37°C and 2 mM CaCl2 and AA at the indicated concentrations were added to start 5-LO product formation. The reaction was stopped after 10 min at 37°C by the addition of 1 ml ice-cold methanol and the formed metabolites were analysed by HPLC as described for intact cells.
Subcellular fractionation by mild detergent lysis
Subcellular localization of 5-LO was investigated by cellular fractionation as described previously (Werz et al., 2001). In brief, freshly isolated PMNL (3 × 107 cells) in 1 ml PGC buffer were incubated at 37°C for 10 min with the indicated stimuli and chilled on ice. Nuclear and non-nuclear fractions were obtained after cell lysis by 0.1% NP-40. Aliquots of these fractions were analysed for 5-LO protein by SDS–PAGE and western blotting.
SDS–PAGE and western blotting
HeLa cells (2 × 106) were re-suspended in 100 μl PGC buffer, 100 μl of ice-cold 2 × SDS–PAGE sample loading buffer (SDS-b; 20 mM Tris-HCl, pH 8, 2 mM EDTA, 5% SDS (wv−1), 10% β-mercaptoethanol) was added, vortexed and heated for 6 min at 95°C. Total HeLa cell lysates or subcellular fractions of PMNL (20 μl, each) were mixed with 4 μl of glycerol/0.1% bromophenol blue (1:1, vv−1) and analysed by SDS–PAGE on a 10% gel. Correct loading of the gel and transfer of proteins to the nitrocellulose membrane was confirmed by Ponceau staining. After electroblot to nitrocellulose membranes (GE Healthcare, Munich, Germany), blocking with 5% non-fat dry milk for 1 h at room temperature (RT), membranes were washed and incubated with primary antibodies (ABs) overnight at 4°C. The polyclonal anti-5-LO antiserum (AK7, 1551, kindly provided by Dr Olof Rådmark, Stockholm, Sweden) was affinity-purified on a 5-LO column and used as 1:10 dilution, polyclonal anti-FLAP antiserum was provided by Dr A Hatzelmann, Konstanz, Germany; and used as 1:1000 dilution. The membranes were washed and incubated with 1:1000 dilution of alkaline phosphatase conjugated IgGs (Sigma) for 2 h at RT. After washing, proteins were visualized with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma).
Indirect immunofluorescence microscopy
Human PMNL (1.5 × 106 cells in 500 μl PGC buffer) were incubated at 37°C for 15 min with licofelone, MK-886 or dimethylsulphoxide (DMSO) (vehicle). Cells were then centrifuged at 30 g for 1 min onto poly-L-lysine (MW 150 000–300 000, Sigma-Aldrich, Deisenhofen, Germany)-coated glass coverslips in the wells of a 12-well plate, and activated by addition of 2.5 μM ionophore A23187, for 3 min at 37°C. Cells were fixed in methanol (−20°C, 30 min) and permeabilized in acetone (−20°C, 3 min), followed by two wash steps with PBS. The staining was performed by incubating the coverslips with the anti-5-LO serum (1551, AK-7) for 30 min at RT. The coverslips were then washed five times with PBS, incubated with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, Karlsruhe, Germany, diluted 1:300 in PBS) for 10 min at RT in the dark, and washed five times with PBS. The DNA was stained with 0.1 μg ml−1 diamidino-2-phenylindole (DAPI) in PBS for 3 min at RT in the dark. The coverslips were then washed two times and mounted on glass slides with Mowiol (Calbiochem, San Diego, CA, USA) containing 2.5% N-propyl gallate (Sigma). The fluorescence was visualized with a Zeiss Axiovert 200 M microscope.
Alignment of licofelone and MK-886
The flexible alignment of the structures of licofelone and MK-886 was performed using MOE 2005.06 (The Chemical Computing Group, Montreal, Canada; www.chemcomp.org). Default options were used, including the standard force field MMFF.94x. For visualization of the pharmacophore features in the alignment of MK-886 und licofelone, an extended version of SQUID was used (Y Tanrikulu et al., unpublished results) (Renner and Schneider, 2004). Potential pharmacophore points (PPPs) are visualized as ellipsoids. Features were clustered into PPPs using following cluster radii: lipophilic PPPs: 1.6 Å, hydrogen-bond donor PPPs: 1.9 Å, hydrogen-bond acceptor PPPs: 2.3 Å. Visualization was performed using PyMOL (DeLano, 2002).
Statistics
Statistical evaluation of the data was performed by one-way ANOVA followed by a Tukey-HSD post hoc test. P<0.05 (*), <0.01 (**) or <0.001 (***) was considered significant.
Reagents
Licofelone was provided by Merckle (Blaubeuren, Germany). MK-886 and BWA4C were generous gifts from Dr AW Ford-Hutchinson, Merck-Frosst (Canada) and from Dr LG Garland (Wellcome Research Laboratories), respectively. DMEM, GibcoBRL, Life Technologies (Rockville, MD, USA); fetal calf serum, Ca2+-ionophore A23187, AA, diamide, DTT, sodium arsenite (SA), Sigma; HPLC solvents, Merck (Darmstadt, Germany).
Results
Licofelone is a potent inhibitor of cellular 5-LO product formation
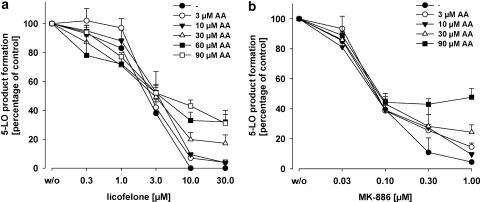
Freshly isolated PMNL were preincubated with licofelone and 5-LO product synthesis was induced by addition of ionophore A23187 (2.5 μM) together with the indicated concentrations of AA as exogenous substrate (Figure 1). Licofelone caused a concentration-dependent inhibition of 5-LO product synthesis for cells stimulated with ionophore alone or in the presence of 3 or 10 μM AA, with consistent IC50 values ranging from 1.7 to 3 μM, and in the absence of exogenous AA complete inhibition of 5-LO activity was achieved at 10 μM licofelone (Figure 1a). However, in the presence of 30, 60 or 90 μM AA, licofelone (even at 30 μM) failed to suppress 5-LO activity completely (about 35% activity still remained), although the IC50 values (3–3.8 μM) at high AA concentrations were quite similar (Figure 1a). We would point out that the high AA concentrations (60 and 90 μM) used are not considered physiologically relevant but instead are applied here for experimental reasons. No significant lysis of PMNL by treatment with 90 μM AA within 30 min was observed (analysed by light microscopy/Trypan blue staining), ensuring cellular integrity (not shown).
Figure 1.
Inhibition of 5-LO product synthesis by licofelone and MK-886 in intact PMNL. Freshly isolated PMNL (7.5 × 106 ml−1 PGC buffer) were preincubated with licofelone (a) or MK-886 (b) as indicated at 37°C. After 15 min, 2.5 μM ionophore plus the indicated concentrations of AA were added and after 10 min, the formation of 5-LO products was determined as described. Values are given as mean+s.e., n=3–4. 5-LO product synthesis in the absence of test compounds (100%, control) was 37.4±3.8 (ionophore), 57.9±5.7 (ionophore+3 μM AA), 105.1±8.4 (ionophore+10 μM AA), 176.6±31.1 (ionophore+30 μM AA), 145.1±24.4 (ionophore+60 μM AA) and 105.8±30.5 (ionophore+90 μM AA) ng per 106 PMNL. AA, arachidonic acid; 5-LO, 5-lipoxygenase; PGC buffer, PBS containing 1 mg ml−1 glucose and 1 mM CaCl2; PMNL, polymorphonuclear leukocytes.
In agreement with our previous studies (Steinhilber et al., 1993; Werz et al., 1997), a similar pattern in 5-LO activity inhibition was found for the FLAP inhibitor MK-886 (Figure 1b). Thus, the IC50 values (70–90 nM) of MK-886 for 5-LO product synthesis in PMNL were only barely affected by increasing the AA concentrations. As observed for licofelone, exogenous AA concentration-dependently reduced maximal inhibition of 5-LO activity also by MK-886 (Figure 1b).
Licofelone and MK-886 share structural properties
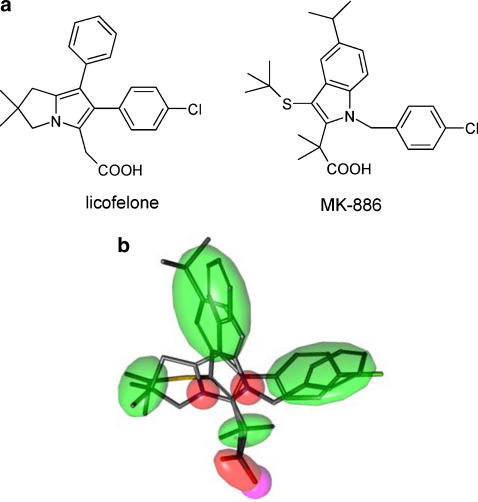
The structures of licofelone and MK-886 (Figure 2a) were compared. Flexible alignment of the two molecules resulted in a superposition with a strain energy (U) of 83, a total mutual similarity score (F) of 99 and an objective function value (S) of 182 (Figure 2b). Matching pharmacophore features can be seen for the aromatic ring systems, and the identical position of the carboxyl groups. Aliphatic moieties are arranged in two lipophilic clusters. These data suggest that MK-886 and licofelone essentially contain the same pharmacophores.
Figure 2.
Comparison of the structures of licofelone and MK-886. (a) Chemical structures of licofelone and MK-886. (b) Flexible alignment of licofelone and MK-886 showing mutual PPPs. Green shapes represent lipophilic features, red ones show potential hydrogen-bond acceptors, and the polar group (carboxy function) is displayed in magenta. PPP, potential pharmacophore point.
Licofelone is a weak inhibitor of 5-LO activity in cell-free assays
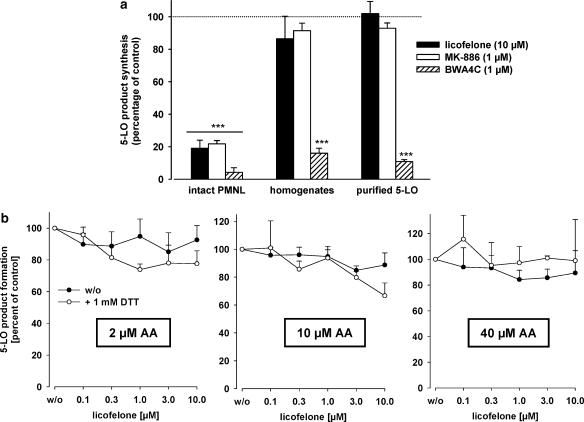
According to its function as a FLAP inhibitor, MK-886 potently suppresses 5-LO activity in intact cells but hardly affects 5-LO activity in broken cell preparations (Dixon et al., 1990), implying that the compound does not directly interfere with 5-LO catalytic activity. The ability of licofelone and of MK-886 to inhibit 5-LO directly was analysed in homogenates of PMNL as well as for purified human recombinant 5-LO (expressed in E. coli). The well-recognized, direct, iron ligand-type 5-LO inhibitor BWA4C (Tateson et al., 1988) was used as a positive control. As shown in Figure 3a, all three inhibitors markedly suppressed 5-LO product synthesis in intact PMNL stimulated by ionophore plus 40 μM AA. However, licofelone (10 μM) and MK-886 (1 μM) failed to suppress significantly the activity of 5-LO in homogenates and of purified 5-LO. No such a difference was apparent for the direct 5-LO inhibitor BWA4C (1 μM) that potently blocked 5-LO activity in cell-free assays.
Figure 3.
Inhibition of 5-LO activity by licofelone in cell-free assays; influence of DTT. (a) Inhibition of the activity of 5-LO in intact PMNL, in homogenates of PMNL, and of purified 5-LO. Intact PMNL were preincubated with the test compounds (or DMSO as vehicle, w/o) for 15 min before addition of 2.5 μM ionophore plus 40 μM AA. For determination of the inhibition of the activity of 5-LO in homogenates or of purified human recombinant 5-LO, test compounds were added to the incubations 5 min before addition of 1 mM Ca2+ plus 40 μM AA. Values are given as mean+s.e., n=4; data were analysed by ANOVA followed by Tukey-HSD post hoc test: ***P<0.001 vs control (vehicle). (b) Influence of DTT on the efficacy of licofelone for 5-LO inhibition in a cell-free assay. Homogenates of 7.5 × 106 PMNL in PG buffer containing 1 mM EDTA were prepared by sonication. ATP (1 mM) and licofelone together with or without 1 mM DTT was added and samples were kept on ice for 5–10 min. Samples were preincubated at 37°C for 30 s before addition of 2, 10 or 40 μM AA plus 2 mM CaCl2, each. After 10 min, 5-LO products formed were determined. Values are given as mean+s.e., n=4; data were analysed by ANOVA followed by Tukey-HSD post hoc test. P>0.05. AA, arachidonic acid; DMSO, dimethylsulphoxide; DTT, dithiothreitol; 5-LO, 5-lipoxygenase; PG buffer, PBS containing 1 mg ml−1 glucose; PMNL, polymorphonuclear leukocytes.
Representatives of the class of nonredox-type 5-LO inhibitors such as ZM230487 or L-739.010 are potent 5-LO inhibitors in intact cells (Werz et al., 1998), whereas for inhibition of crude 5-LO in cell-free systems, substantially higher concentrations are required due to high peroxide levels. However, reconstitution of glutathione peroxidase (GPx) activity by inclusion of thiols restores the potent inhibition of 5-LO by ZM230487 or L-739.010 (Werz et al., 1998). It appeared reasonable that licofelone could act in a similar manner as ZM230487 or L-739.010 and thus potently inhibit 5-LO after supplementation of homogenates with thiols. As shown in Figure 3b, addition of exogenous DTT did not significantly restore 5-LO inhibition by licofelone, regardless of the AA concentrations. In other experiments, addition of DTT also did not enable MK-886 to block 5-LO activity in homogenates, as expected (data not shown). In summary, under reducing conditions and low AA concentrations licofelone (up to 10 μM) exerted no significant, direct inhibition of 5-LO in cell-free assays.
The potency of licofelone depends on the stimulus to activate 5-LO
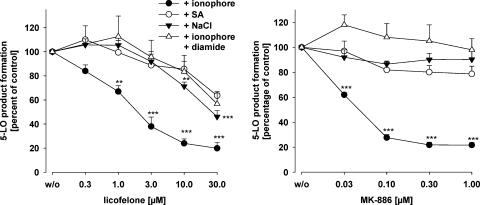
Next, the efficacies of licofelone and MK-886 were tested for 5-LO inhibition in PMNL under conditions where 5-LO was either activated by Ca2+-dependent pathways using ionophore or by cell stress such as genotoxic stress using sodium arsenite (SA, 10 μM), or hyperosmotic stress (300 mM NaCl), each in the presence of 40 μM AA. Moreover, oxidative stress using diamide (500 μM) was applied to check if the compounds are influenced by an elevated peroxide tone. For both compounds, a comparable pattern of inhibition of 5-LO product formation was evident. Whereas ionophore-induced 5-LO activation was strongly inhibited by MK-886 or licofelone (IC50=60 nM and 2.3 μM, respectively), 5-LO activity evoked by hyperosmotic (NaCl) or genotoxic (SA) stress was not or barely suppressed by MK-886 up to 1 μM, and also the potency of licofelone was strongly impaired (IC50 values ⩾30 μM) under cell stress conditions (Figure 4). Moreover, elevation of the peroxide tone (using diamide) strongly reduced the efficacy of both compounds in ionophore-stimulated cells.
Figure 4.
The potency of licofelone depends on the stimulus to activate 5-LO. PMNL (7.5 × 106 in 1 ml PGC buffer, final volume) were preincubated with licofelone or MK-886 (or DMSO as vehicle, w/o) as indicated at 37°C. After 12 min, cells were pretreated with 10 μM SA, 500 μM diamide or 300 mM NaCl or left untreated. After 3 min untreated and diamide-treated cells were stimulated with 2.5 μM ionophore plus 40 μM AA, and SA- or NaCl-treated cells were supplemented with 40 μM AA. After another 10 min, the formation of 5-LO products was determined. 5-LO product synthesis in the absence of test compounds (100%, control) was 177.1 ± 20.2 (ionophore+AA), 145.0±56.0 (ionophore+diamide+AA), 55.4±7.6 (SA+AA), and 61.7±11.8 (NaCl+AA) ng per 106 PMNL. Values are given as mean+s.e., n=4–5; data were analysed by ANOVA followed by Tukey-HSD post hoc test: **P<0.01, ***P<0.001 vs vehicle control (w/o). AA, arachidonic acid; DMSO, dimethylsulphoxide; 5-LO, 5-lipoxygenase; PGC buffer, PBS containing 1 mg ml−1 glucose and 1 mM CaCl2; PMNL, polymorphonuclear leukocytes; SA, sodium arsenite.
Licofelone inhibits translocation of 5-LO to the nuclear membrane
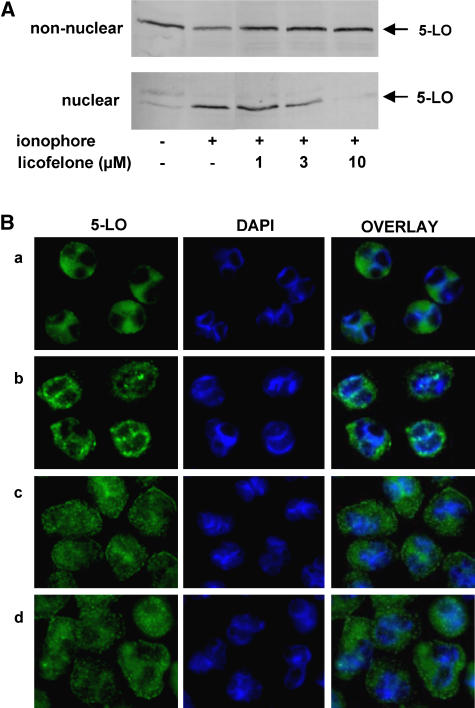
Depending on the experimental conditions and settings, FLAP inhibitors, such as MK-886 were found to prevent 5-LO translocation to the nuclear membrane (Rouzer et al., 1990; Kargman et al., 1991; Brideau et al., 1992), whereas in some studies, in particular when FLAP is not operative, MK-886 failed in this respect (Kargman et al., 1992; Brock et al., 1998). First, we investigated if licofelone inhibits ionophore-induced 5-LO translocation by means of subcellular fractionation using mild detergent lysis and detection of 5-LO by western blotting. PMNL were preincubated with licofelone and then stimulated with ionophore to induce 5-LO redistribution from the cytosol to the nuclear membrane. After subcellular fractionation, the nuclear and the non-nuclear (cytosolic) fraction was examined for 5-LO protein by western blotting. As shown in Figure 5A, in resting PMNL, 5-LO is exclusively in the non-nuclear fraction but stimulation with ionophore causes accumulation of 5-LO in the nuclear fraction. Licofelone concentration-dependently suppressed ionophore-evoked 5-LO redistribution.
Figure 5.
Licofelone inhibits translocation of 5-LO to the nuclear membrane. (A) Freshly isolated PMNL (3 × 107 ml-1 PGC buffer) were preincubated with licofelone as indicated at 37°C. After 15 min, 2.5 μM ionophore was added and after 10 min, the samples were chilled on ice. After detergent (0.1% NP-40) lysis and subcellular fractionation, 5-LO was determined in nuclear and non-nuclear fractions by western blotting. The results shown are representative of at least three independent experiments. (B) Indirect immunofluorescence microscopy. PMNL were preincubated with licofelone (10 μM) or MK-886 (300 nM) or vehicle (DMSO) as indicated, centrifuged onto poly-L-lysine-coated glass coverslips and activated by ionophore A23187 (2.5 μM) as indicated. After 3 min, cells were fixed, permeabilized and incubated with anti-5-LO serum (1551, AK-7). After addition of Alexa Fluor 488 goat anti-rabbit IgG the fluorescence was analysed. Left panel: staining for 5-LO; middle panel: staining for nuclei (DAPI); right panel: overlay of 5-LO and nuclear staining. (a) Vehicle, (b) vehicle+A23187, (c) licofelone+A23187, (d) MK-886+A23187. The photographs shown are representative of four similar samples. DAPI, diamidino-2-phenylindole; DMSO, dimethylsulphoxide; G; 5-LO, 5-lipoxygenase; PGC buffer, PBS containing 1 mg ml−1 glucose and 1 mM CaCl2; PMNL, polymorphonuclear leukocytes.
Next, 5-LO redistribution was analysed in intact PMNL by indirect immunofluorescence microscopy. In agreement with others (Brock et al., 1997), in resting cells (a), 5-LO was homogenously distributed in the cytosol outside the nucleus, whereas upon ionophore stimulation (b) the major portion of 5-LO was localized at the nuclear envelope yielding a granular pattern (Figure 5B). Pretreatment with either licofelone (c) or MK-886 (d) blocked the accumulation of 5-LO at the nuclear envelope, but a homogenous cytosolic 5-LO distribution as observed for resting cells was not retained, instead 5-LO appeared to agglomerate in non-nuclear regions, in the cytosol and close to the plasma membrane.
Licofelone is a potent inhibitor of FLAP-mediated 5-LO product formation
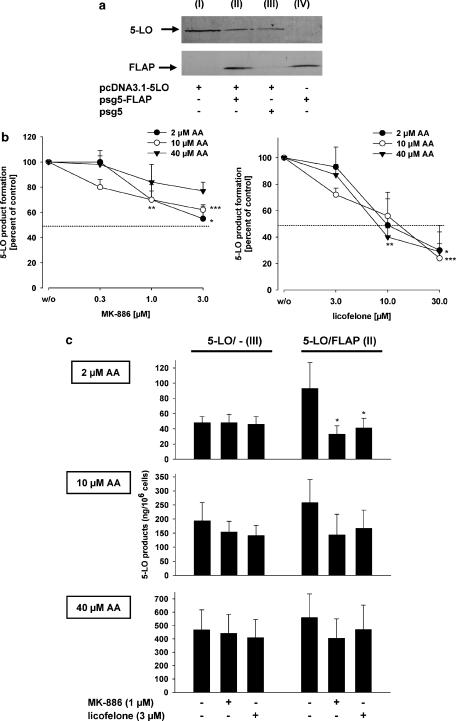
To investigate further the role of FLAP in inhibition of cellular 5-LO product synthesis by licofelone, HeLa cells that are essentially deficient in 5-LO and FLAP (Figure 6a), were either co-transfected with 5-LO plus FLAP or transfected with 5-LO alone plus the empty vector plasmid (pSG5, to adjust correct plasmid concentrations to normalize expression of 5-LO protein). Expression of proteins was assessed by western blotting (Figure 6a). First, HeLa cells transfected with the 5-LO plasmid plus the control plasmid pSG5 (no FLAP) were analysed. Cells were preincubated with MK-886 or licofelone and stimulated with 10 μM ionophore (optimized concentration) plus 2, 10 or 40 μM AA. As expected, MK-886 hardly suppressed 5-LO product formation in HeLa cells lacking FLAP (IC50>3 μM), compared to PMNL (IC50=60 nM). Nevertheless, 3 μM MK-886 reduced 5-LO activity by about 23–45% (Figure 6b). Also for licofelone, much higher concentrations were needed to block 5-LO product synthesis in FLAP-devoid HeLa cells (IC50 approximately 10 μM, Figure 6b) vs PMNL (IC50 approximately 2 μM, see Figure 1a), and the potency was not influenced by the AA concentration.
Figure 6.
Licofelone is a potent inhibitor of FLAP-mediated 5-LO product formation. HeLa cells were transiently transformed with plasmids pcDNA3.1–5LO, pSG5-FLAP and pSG5 empty vector (10 μg, each) as indicated. (a) Expression of 5-LO and FLAP. HeLa cells (2 × 106) were re-suspended in 100 μl PGC buffer, mixed with the same volume of SDS-b and heated for 6 min at 95°C. Samples were analysed by western blotting for 5-LO and FLAP proteins. (b) HeLa cells (2 × 106) transfected with 5-LO alone were re-suspended in 1 ml PGC buffer and incubated with licofelone or MK-886 (or DMSO as vehicle, w/o) as indicated. After 15 min at 37°C, cells were stimulated with 5 μM ionophore plus 2, 10 or 40 μM AA for another 10 min. 5-LO products were extracted and determined by HPLC. (c) HeLa cells (2 × 106) transfected with 5-LO plus pSG5 empty vector (5-LO/- (3)) or with 5-LO plus FLAP (5-LO/FLAP (2)) were re-suspended in 1 ml PGC buffer and incubated with 3 μM licofelone or 1 μM MK-886 (or DMSO as vehicle, w/o) as indicated. After 15 min at 37°C, cells were stimulated with 5 μM ionophore plus 2, 10 or 40 μM AA for another 10 min. 5-LO products were extracted and determined by HPLC. Results are given as mean+s.e., n=3–4; data were analysed by ANOVA followed by Tukey-HSD post hoc test: *P<0.05, **P<0.01, ***P<0.001 vs vehicle control (w/o). AA, arachidonic acid; DMSO, dimethylsulphoxide; FLAP, 5-lipoxygenase-activating protein; 5-LO, 5-lipoxygenase; PGC buffer, PBS containing 1 mg ml−1 glucose and 1 mM CaCl2; SDS-b, 2 × sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) sample loading buffer.
Next, the potencies of MK-886 and licofelone were compared by assessing 5-LO inhibition in HeLa cells expressing 5-LO alone, and in cells expressing both 5-LO and FLAP. Again, cells were stimulated with ionophore plus exogenous AA at 2, 10 or 40 μM. In the presence of 2 μM AA, ionophore-induced 5-LO product synthesis in FLAP-devoid HeLa cells was hardly inhibited by 1 μM MK-886 or 3 μM licofelone (Figure 6c). HeLa cells expressing FLAP caused a 2.4-fold increase in 5-LO product synthesis at 2 μM AA. The 5-LO activity in homogenates was about the same for FLAP-positive and FLAP-negative HeLa cells (not shown). Of interest, this upregulated 5-LO activity (due to the presence of FLAP) was potently suppressed by both MK-886 and licofelone at 1 and 3 μM, respectively. On the other hand, in the presence of 10 or 40 μM AA, co-expression of FLAP caused only modest enhancement of ionophore-induced 5-LO product synthesis, and the inhibition of 5-LO activity by MK-886 or licofelone was not significant.
Discussion
The objective of the present study was to elucidate the mechanism of licofelone-mediated inhibition of LT synthesis. Because models with intact cells and measurement of drug-mediated effects on LT release do not allow an unequivocal identification of the mechanism of action, that is, 5-LO or FLAP inhibition, a series of experiments with intact cells and cell-free test systems were performed. The results indicate that licofelone requires the cellular environment to suppress 5-LO activity potently, that is, when FLAP is operative, and we conclude that licofelone acts on FLAP. This hypothesis is based on the following findings: (i) licofelone potently inhibited ionophore-induced 5-LO product synthesis in intact FLAP-expressing cells but was barely effective in cell-free assays. (ii) The potency of licofelone in PMNL was impaired in the presence of exogenous AA, comparable to the effects of MK-886 known to compete with AA for binding to FLAP (Mancini et al., 1993). (iii) Licofelone as well as MK-886 failed to inhibit potently cell stress-induced 5-LO product synthesis in PMNL. (iv) Treatment of PMNL with either licofelone or MK-886, before addition of ionophore prevented nuclear localization and induced the same 5-LO subcellular redistribution pattern. (v) At low concentrations, licofelone and MK-886 had modest effects on cellular 5-LO activity in HeLa cells lacking FLAP, whereas elevated 5-LO product formation due to FLAP was potently repressed. Finally, licofelone shared structural and chemical similarities with MK-886.
Previous reports have documented that licofelone potently inhibits cellular 5-LO activity in various cells. Thus, the IC50 values were determined at 0.23 μM in human granulocytes (Laufer et al., 1994b), 3.6 μM in rat basophilic leukaemia (RBL)-1 cells (Tries et al., 2002), and 3.8 μM in mixed PMNL/platelet suspensions (Rotondo et al., 2002), which is in good agreement with the efficacy of licofelone in our hands (IC50=1.7 μM) under same experimental conditions. In all these studies, ionophore A23187-stimulated leukocytes expressing FLAP were used as the test system, a common assay for screening and evaluation of LT synthesis inhibitors (Werz and Steinhilber, 2005). Under such conditions, FLAP inhibitors such as MK-886 or BayX-1005 are among the most potent inhibitors of LT biosynthesis (IC50=2.5 and 26 nM; Gillard et al., 1989; Fruchtmann et al., 1993). However, FLAP inhibitors, assumed to interrupt the transfer of AA to 5-LO, lose efficacy when excess of AA is provided (Steinhilber et al., 1993; Werz et al., 1997). Licofelone was also less active when exogenous AA was supplemented, as was MK-886. In this respect, the poor effectiveness of FLAP inhibitors in whole blood assays (IC50=1.1 μM for MK-886) has been explained by high concentrations of fatty acids present (Werz, 2002). Similarly, in the presence of licofelone, LTC4 levels in stimulated human whole blood were similar to those determined in positive controls (Tries et al., 2002). However, licofelone is also an inhibitor of COX and, as demonstrated with indomethacin, COX inhibition in stimulated whole blood resulted in a shift of AA metabolism and LTC4 levels were 50% higher when compared with positive controls and assays with licofelone.
Another property of licofelone shared with MK-886 is the reduced efficacy of inhibition of cell stress-induced 5-LO product formation. Cell stress activates cellular 5-LO by Ca2+-independent pathways involving phosphorylation of the 5-LO enzyme at Ser-271 by mitogen-activated protein kinase-activated protein kinase-2 (MAPKAPK-2) (Werz et al., 2002a, 2002b). Under such conditions and also when the peroxide tone is increased, the nonredox-type 5-LO inhibitors ZM230487 and L739.010, but not the iron ligand BWA4C are about 100-fold less potent, compared to stimulation with ionophore (Werz et al., 1998; Fischer et al., 2003, 2004). Therefore, a stimulus-dependent inhibition of 5-LO may also apply to the FLAP inhibitor MK-886. One may speculate that induction of 5-LO product synthesis by cell stress via MAPKAPK-2 does not involve FLAP, and 5-LO (in the phosphorylated state) may be active at another subcellular locus. Alternatively, cell stress may render FLAP insensitive towards its inhibitors. The respective molecular interrelations are under investigation in our laboratories.
Among the blockers of 5-LO product synthesis, FLAP inhibitors were reported to prevent translocation of 5-LO to the nuclear membrane where FLAP resides (Rouzer et al., 1990; Evans et al., 1991), although in some reports MK-886 failed in this respect, seemingly depending on the presence (Kargman et al., 1992) or participation (Brock et al., 1998) of FLAP in the 5-LO activation process. Licofelone blocked 5-LO nuclear translocation at reasonable concentrations. Indirect immunofluorescence microscopy showed that licofelone and MK-886 not only prevented accumulation of 5-LO at the nuclear envelope, but also caused the same pattern of 5-LO redistribution, that is 5-LO agglomeration in non-nuclear regions. Translocation of 5-LO to the nuclear membrane and interaction with FLAP is believed to be crucial for 5-LO to access its substrate. However, when there is ample supply of substrate in the cytosol, for example upon addition of exogenous AA, nuclear membrane localization of 5-LO and thus the necessity of FLAP might be dispensable for 5-LO product formation (Werz et al., 2001). This may also explain the loss of efficacy of licofelone and MK-886 in the presence of exogenous AA observed in PMNL and HeLa cells expressing FLAP. Notably, in HeLa cells devoid of FLAP, variation of the AA concentration did not affect the efficacy of licofelone or MK-886.
In cell-free assays, licofelone or MK-886 failed to inhibit potently 5-LO activity. Such a loss of efficacy, compared to intact cells, was observed not only for FLAP inhibitors (Dixon et al., 1990) but also for the nonredox-type 5-LO inhibitors ZD2138, ZM230487, L-739.010 and CJ-13610 (Smith et al., 1995; Werz et al., 1997, 1998; Fischer et al., 2004). Low peroxide levels achieved by reconstitution of GPx activity restored potent 5-LO inhibition of the nonredox-type inhibitors in cell-free assays (Werz et al., 1998; Fischer et al., 2004). For licofelone, only a slightly increased efficacy in PMNL homogenates was evident upon supplementation with DTT, at least at low AA concentrations. However, when this very weak effect of DTT on the action of licofelone is compared with the strong impact of thiols on the efficacy of nonredox-type 5-LO inhibitors, licofelone obviously does not behave like a typical nonredox-type inhibitor.
The hypothesis that FLAP is instrumental in the suppression of 5-LO product synthesis by licofelone is strengthened by our 5-LO/FLAP co-transfection experiments. Thus, licofelone and MK-886 moderately inhibited 5-LO product synthesis in HeLa cells expressing only 5-LO. However, the upregulated 5-LO product formation due to coexpression of FLAP was efficiently blocked by both licofelone and MK-886. Increasing the AA concentration blunted the stimulatory effect of FLAP coexpression, and licofelone or MK-886 hardly suppressed 5-LO product formation under these conditions. Analysis of the functionality of MK-886 in osteosarcoma cells expressing 5-LO together with or without FLAP gave comparable results (Kargman et al., 1992). It must be pointed out that licofelone and MK-886 at higher concentrations both significantly inhibited 5-LO product synthesis in HeLa cells devoid of FLAP. This effect was more pronounced for licofelone as compared to MK-886. We conclude that besides FLAP, certain factors or components (for example, GPxs but also others) within the intact cellular environment seemingly govern 5-LO inhibition by licofelone and also by MK-886.
Taken together, licofelone efficiently reduced 5-LO product synthesis in intact cells, presumably by interference with FLAP, but exerted only weak inhibitory actions on the 5-LO enzyme directly. Licofelone is a representative of the so-called ‘dual inhibitors' of the COX and 5-LO pathway that have proven to be efficient in various preclinical animal models of inflammation and in clinical studies on human subjects. Several reports have documented efficient suppression of LT levels by licofelone in patients (Ding and Cicuttini, 2003) and licofelone is currently in Phase III of clinical development for treatment of osteoarthritis. The finding that licofelone apparently interferes with COX-1 and -2, 5-LO and also with FLAP is not surprising since all these enzymes bind the same molecule namely AA, and in view of the fatty acid-like structure of licofelone, it is reasonable to speculate that this compound targets multiple enzymes possessing an AA-binding site.
Acknowledgments
We thank Sven George for expert technical assistance. This study was supported by Merckle (Blaubeuren, Germany).
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenase
- DTT
dithiothreitol
- FLAP
5-lipoxygenase-activating protein
- GPx
glutathione peroxidase
- LO
lipoxygenase
- LT
leukotriene
- MAPKAPK
mitogen-activated protein kinase-activated protein kinase
- PBS
phosphate-buffered saline pH 7.4
- PG
prostaglandin
- PG buffer
PBS containing 1 mg ml−1 glucose
- PGC buffer
PBS containing 1 mg ml−1 glucose and 1 mM CaCl2
- PMNL
polymorphonuclear leukocytes
- PPP
potential pharmacophore points
- SA
sodium arsenite
- SDS-b
2 × sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) sample loading buffer
Conflict of interest
The authors state no conflict of interest.
References
- Abramovitz M, Wong E, Cox ME, Richardson CD, Li C, Vickers PJ. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur J Biochem. 1993;215:105–111. doi: 10.1111/j.1432-1033.1993.tb18012.x. [DOI] [PubMed] [Google Scholar]
- Brideau C, Chan C, Charleson S, Denis D, Evans JF, Ford-Hutchinson AW, et al. Pharmacology of MK-0591 (3-[1-(4-chlorobenzyl)-3-(t-butylthio)-5-(quinolin-2-yl-methoxy)-indol-2-yl]-2,2-dimethyl propanoic acid), a potent, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1992;70:799–807. doi: 10.1139/y92-107. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Bailie MB, Peters-Golden M. Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J Biol Chem. 1997;272:8276–8280. doi: 10.1074/jbc.272.13.8276. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Capacity for repeatable leukotriene generation after transient stimulation of mast cells and macrophages. Biochem J. 1998;329:519–525. doi: 10.1042/bj3290519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brungs M, Rådmark O, Samuelsson B, Steinhilber D. Sequential induction of 5-lipoxygenase gene expression and activity in Mono Mac 6 cells by transforming growth factor-beta and 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1995;92:107–111. doi: 10.1073/pnas.92.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrum RS, Goulet JL, Griffiths RJ, Koller BH. Role of the 5-lipoxygenase-activating protein (FLAP) in murine acute inflammatory responses. J Exp Med. 1997;185:1065–1075. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti F, Laufer S. Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res. 2001;43:429–436. doi: 10.1006/phrs.2000.0784. [DOI] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific: San Carlos, CA, USA; 2002. [Google Scholar]
- Ding C, Cicuttini F. Licofelone (Merckle) IDrugs. 2003;6:802–808. [PubMed] [Google Scholar]
- Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- Evans JF, Leveille C, Mancini JA, Prasit P, Therien M, Zamboni R, et al. 5-lipoxygenase-activating protein is the target of a quinoline class of leukotriene synthesis inhibitors. Mol Pharmacol. 1991;40:22–27. [PubMed] [Google Scholar]
- Fischer L, Steinhilber D, Werz O. Molecular pharmacological profile of the nonredox-type 5-lipoxygenase inhibitor CJ-13,610. Br J Pharmacol. 2004;142:861–868. doi: 10.1038/sj.bjp.0705860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Szellas D, Rådmark O, Steinhilber D, Werz O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003;17:949–951. doi: 10.1096/fj.02-0815fje. [DOI] [PubMed] [Google Scholar]
- Fruchtmann R, Mohrs KH, Hatzelmann A, Raddatz S, Fugmann B, Junge B, et al. In vitro pharmacology of BAY X1005, a new inhibitor of leukotriene synthesis. Agents Actions. 1993;38:188–195. doi: 10.1007/BF01976210. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gillard J, Ford-Hutchinson AW, Chan C, Charleson S, Denis D, Foster A, et al. L-663,536 (MK-886) (3-(1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl)-2,2-dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989;67:456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Kargman S, Prasit P, Evans JF. Translocation of HL-60 cell 5-lipoxygenase—inhibition of A23187 or N-formyl-methionyl-leucyl-phenylalanine-induced translocation by indole and quinoline leukotriene synthesis inhibitors. J Biol Chem. 1991;266:23745–23752. [PubMed] [Google Scholar]
- Kargman S, Vickers PJ, Evans JF. A23187-induced translocation of 5-lipoxygenase in osteosarcoma cells. J Cell Biol. 1992;119:1701–1709. doi: 10.1083/jcb.119.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer S, Tries S, Augustin J, Dannhardt G. Pharmacological profile of a new pyrrolizine derivative inhibiting the enzymes cyclo-oxygenase and 5-lipoxygenase. Arzneimittelforschung. 1994a;44:629–636. [PubMed] [Google Scholar]
- Laufer SA, Augustin J, Dannhardt G, Kiefer W. 6,7-Diaryldihydropyrrolizin-5-yl)acetic acids, a novel class of potent dual inhibitors of both cyclooxygenase and 5-lipoxygenase. J Med Chem. 1994b;37:1894–1897. doi: 10.1021/jm00038a021. [DOI] [PubMed] [Google Scholar]
- Mancini JA, Abramovitz M, Cox ME, Wong E, Charleson S, Perrier H, et al. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993;318:277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- Miller DK, Gillard JW, Vickers PJ, Sadowski S, Léveillé C, Mancini JA, et al. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Provost P, Doucet J, Hammarberg T, Gerisch G, Samuelsson B, Rådmark O. 5-Lipoxygenase interacts with coactosin-like protein. J Biol Chem. 2001;276:16520–16527. doi: 10.1074/jbc.M011205200. [DOI] [PubMed] [Google Scholar]
- Renner S, Schneider G. Fuzzy pharmacophore models from molecular alignments for correlation-vector-based virtual screening. J Med Chem. 2004;47:4653–4664. doi: 10.1021/jm031139y. [DOI] [PubMed] [Google Scholar]
- Rotondo S, Dell'Elba G, Krauze-Brzosko K, Manarini S, Martelli N, Pecce R, et al. Licofelone, a dual lipoxygenase–cyclooxygenase inhibitor, downregulates polymorphonuclear leukocyte and platelet function. Eur J Pharmacol. 2002;453:131–139. doi: 10.1016/s0014-2999(02)02385-3. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Ford-Hutchinson AW, Morton HE, Gillard JW. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore challenged leukocytes. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- Smith WG, Shaffer AF, Currie JL, Thompson JM, Kim S, Rao T, et al. Characterization of 5-lipoxygenase inhibitors in biochemical and functional in vivo assays. J Pharmacol Exp Ther. 1995;275:1332–1338. [PubMed] [Google Scholar]
- Steinhilber D, Hoshiko S, Grunewald J, Rådmark O, Samuelsson B. Serum factors regulate 5-lipoxygenase activity in maturating HL60 cells. Biochim Biophys Acta. 1993;1178:1–8. doi: 10.1016/0167-4889(93)90104-w. [DOI] [PubMed] [Google Scholar]
- Tateson JE, Randall RW, Reynolds CH, Jackson WP, Bhattacherjee P, Salmon JA, et al. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br J Pharmacol. 1988;94:528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tries S, Neupert W, Laufer S. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. Inflamm Res. 2002;51:135–143. doi: 10.1007/pl00000285. [DOI] [PubMed] [Google Scholar]
- Werz O. 5-Lipoxygenase: cellular biology and molecular pharmacology. Curr Drug Targets—Inflamm Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- Werz O, Burkert E, Samuelsson B, Rådmark O, Steinhilber D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood. 2002a;99:1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- Werz O, Klemm J, Samuelsson B, Rådmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97:2487–2495. doi: 10.1182/blood.v97.8.2487. [DOI] [PubMed] [Google Scholar]
- Werz O, Schneider N, Brungs M, Sailer ER, Safayhi H, Ammon HPT, et al. A test system for leukotriene synthesis inhibitors based on the in-vitro differentiation of the human leukemic cell lines HL-60 and Mono Mac 6. Naunyn Schmied Arch Pharmacol. 1997;356:441–445. doi: 10.1007/pl00005074. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Development of 5-lipoxygenase inhibitors—lessons from cellular enzyme regulation. Biochem Pharmacol. 2005;70:327–333. doi: 10.1016/j.bcp.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Selenium-dependent peroxidases suppress 5-lipoxygenase activity in B-lymphocytes and immature myeloid cells—the presence of peroxidase-insensitive 5-lipoxygenase activity in differentiated myeloid cells. Eur J Biochem. 1996;242:90–97. doi: 10.1111/j.1432-1033.1996.0090r.x. [DOI] [PubMed] [Google Scholar]
- Werz O, Szellas D, Henseler M, Steinhilber D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol Pharmacol. 1998;54:445–451. doi: 10.1124/mol.54.2.445. [DOI] [PubMed] [Google Scholar]
- Werz O, Szellas D, Steinhilber D, Rådmark O. Arachidonic acid promotes phosphorylation of 5-lipoxygenase at Ser-271 by MAPK-activated protein kinase 2 (MK2) J Biol Chem. 2002b;277:14793–14800. doi: 10.1074/jbc.M111945200. [DOI] [PubMed] [Google Scholar]