Abstract
Background and purpose:
The present study addressed the effects of the investigational PDE4 inhibitor roflumilast on leukocyte-endothelial cell interactions and endothelial permeability in vivo and in vitro.
Experimental approach:
In vivo, intravital video-microscopy was used to determine effects of roflumilast p.o. on leukocyte-endothelial cell interactions and microvascular permeability in rat mesenteric venules. In vitro, the effects of roflumilast N-oxide, the active metabolite of roflumilast in humans, and other PDE4 inhibitors on neutrophil adhesion to tumour necrosis factor α (TNFα)-activated human umbilical vein endothelial cells (HUVEC), E-selectin expression and thrombin-induced endothelial permeability was evaluated. Flow cytometry was used to determine the effect of roflumilast on N-formyl-methionyl-leucyl-phenylalanine (fMLP)-induced CD11b upregulation on human neutrophils.
Key results:
In vivo, roflumilast, given 1 h before lipopolysaccharide (LPS), dose-dependently reduced leukocyte-endothelial cell interactions in rat mesenteric postcapillary venules. It also diminished histamine-induced microvascular permeability. Immunohistochemical analyses revealed that roflumilast prevented LPS-induced endothelial P- and E-selectin expression. In vitro, roflumilast N-oxide concentration-dependently suppressed neutrophil adhesion to TNFα-activated HUVEC and CD11b expression on fMLP-stimulated neutrophils. It also reduced TNFα-induced E-selectin expression on HUVEC, when PDE3 activity was blocked. HUVEC permeability elicited by thrombin was concentration-dependently suppressed by roflumilast N-oxide. While roflumilast N-oxide was as potent as roflumilast at inhibiting stimulated endothelial cell and neutrophil functions, both compounds were significantly more potent than the structurally unrelated PDE4 inhibitors, rolipram or cilomilast.
Conclusions and implications:
These findings further support earlier observations on the inhibition of inflammatory cell influx and protein extravasation by roflumilast in vivo.
Keywords: roflumilast, PDE4, leukocyte–endothelial interaction, endothelial permeability, intravital microscopy, E-selectin, P-selectin
Introduction
Over the past decade, inhibition of phosphodiesterase 4 (PDE4) has evolved as a novel approach to the treatment of a myriad of chronic inflammatory ailments ranging from chronic obstructive pulmonary disease (COPD) and asthma to multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease (Sommer et al., 1995; Ross et al., 1997; Banner and Trevethick, 2004; Vignola, 2004; Houslay et al., 2005).
Roflumilast is an oral, once daily investigational PDE4 inhibitor in advanced clinical development for respiratory diseases, such as COPD (Giembycz, 2005; Rabe et al., 2005; Boswell-Smith and Page, 2006). Previous in vitro and in vivo studies revealed the extensive anti-inflammatory potential of roflumilast (Bundschuh et al., 2001; Hatzelmann and Schudt, 2001; Kumar et al., 2003; Jones et al., 2005; Martorana et al., 2005; Mata et al., 2005; Burgess et al., 2006; Growcott et al., 2006; Wollin et al., 2006). Roflumilast reduces antigen-induced inflammatory cell influx and protein accumulation or lipopolysaccharide (LPS)-induced neutrophil influx in bronchoalveolar lavage fluid of Brown–Norway rats in vivo (Bundschuh et al., 2001; Wollin et al., 2003).
Activation of endothelial cells and leukocytes is a hallmark of inflammation that elicits an increase in leukocyte–endothelial interactions and endothelial permeability. Tissue infiltration of leukocytes is preceded by their recruitment from postcapillary venules occurring as a multistep process, initiated by rolling and followed by endothelial adhesion and emigration. These events are orchestrated by sequential expression of cell adhesion molecules (CAMs) on both leukocytes and endothelial cells (Springer, 1994; Kubes and Kerfoot, 2001). Inhibition of PDE4 suppresses leukocyte–endothelial interactions and downregulates CAMs (Sanz et al., 2002, 2005a). Indeed, intravital videomicroscopy of rat mesenteric postcapillary venules revealed that in vivo rolipram (29 μmol kg−1 i.p.) diminishes LPS-induced rapid (0–60 min) or subacute (4 h) leukocyte rolling, adhesion and emigration. In parallel, microvascular P- and E-selectin expressions are abolished (Sanz et al., 2002). In vitro, rolipram reduces neutrophil surface CD11b/CD18 (αMβ2), (Derian et al., 1995; Berends et al., 1997; Sato et al., 2002), tumour necrosis factor-α (TNFα)-induced E-selectin on endothelial cells (Morandini et al., 1996; Blease et al., 1998) or neutrophil adhesion to endothelial cells (Derian et al., 1995; Blease et al., 1998; Jones et al., 2005). Furthermore, enhanced microvascular permeability caused by endothelial cell activation is reversed by cAMP and PDE4 inhibitors (Ortiz et al., 1993; Raeburn et al., 1994; Suttorp et al., 1996).
The present paper describes dose-dependent effects of the PDE4 inhibitor roflumilast on leukocyte rolling, adhesion and emigration at 4 h after stimulation with LPS in rat mesenteric postcapillary venules in vivo, using intravital videomicroscopy (Harris et al., 1994; Johnston et al., 1997; Kubes and Kerfoot, 2001; Sanz et al., 2005a, 2005b). In parallel, the potency and efficacy of roflumilast to inhibit histamine-induced microvascular permeability in rat mesenteric microcirculation was addressed. These in vivo studies were complemented by in vitro investigations exploring direct effects of PDE4 inhibitors, in particular roflumilast N-oxide, on endothelial cells (E-selectin expression, permeability), neutrophils (surface CD11b expression) and neutrophil adherence to endothelial cells. Roflumilast N-oxide is the active metabolite that largely determines the pharmacodynamic activity of roflumilast in rats and in humans (Hatzelmann and Schudt, 2001; Bethke et al., 2007). Our results support the conclusion that roflumilast decreased endothelial cell and leukocyte activation both in vivo and in vitro.
Materials and methods
Animals
This study adhered to the European Community (Directive 86/609/EEC) and Spanish guidelines for the use of experimental animals and it was approved by the institutional committee of animal care and research. Pathogen-free male Sprague–Dawley rats (200–250 g) were acquired from Charles River, Barcelona, Spain and located at the ‘Research Central Unit' of the Faculty of Medicine, University of Valencia under standard conditions.
Intravital microscopy
The details of the experimental preparation have been described previously (Sanz et al., 2002). Briefly, male Sprague–Dawley rats (200–250 g) were anesthetized with sodium pentobarbital (65 mg kg−1, i.p.) and the trachea, right jugular vein and carotid artery were cannulated. After performing a midline abdominal incision, a segment of the midjejunum was exteriorized and placed over an optically clear viewing pedestal maintained at 37°C. The exposed mesentery was continuously superfused with warmed bicarbonate-buffered saline equilibrated with 5% CO2 in nitrogen. An orthostatic microscope (Nikon Optiphot-2, SMZ1) equipped with × 20 objective lens (Nikon SLDW) and × 10 eyepiece permitted tissue visualization. A video camera (Sony SSC-C350P) mounted on the microscope transferred images onto a colour monitor (Sony Trinitron PVM-14N2E) and these images were captured on videotape (Sony SVT-S3000P) for playback analysis (final magnification of the video screen was × 1300). Single unbranched mesenteric venules (25–40 μm diameter) were selected and the diameters measured on-line using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX, USA). Centerline red blood cell velocity (Vrbc) was also measured on-line with an optical Doppler velocimeter (Microcirculation Research Institute). Venular blood flow and wall shear rate were calculated as described previously (House and Lipowsky, 1987). The number of rolling, adherent and emigrated leukocytes was determined off-line during playback analysis of videotaped images.
To determine the effect of roflumilast on leukocyte infiltration elicited by LPS, roflumilast was given at single oral doses of 0.1–1 μmol kg−1. One hour later, 5 ml of LPS (0.2 μg ml−1) was injected i.p. In the control groups, rats received the same volume of saline for the same period of time. After 4 h of LPS or saline administration measurements of mean arterial blood pressure (MABP), Vrbc, vessel diameter, shear rate, leukocyte rolling flux and velocity as well as leukocyte adhesion and emigration were performed.
Immunohistochemistry
Immunohistochemistry was used to examine the expression of P- and E-selectin in rat mesenterial microvessels. Once the experiment using intravital microscopy was completed, the portion exposed to saline or LPS for 4 h with or without roflumilast (10 μmol kg−1) pretreatment was then isolated and fixed in 4% paraformaldehyde for 90 min at 4°C as described previously (Sanz et al., 2002). Immunohistochemical localization of P- and E-selectin was accomplished using a modified avidin and biotin immunoperoxidase technique as previously described by Sanz et al. (2002). Tissue sections were incubated with the anti-rat-P-selectin monoclonal antibody (mAb) (RMP-1) or with the anti-rat-E-selectin mAb (RME-1) for 24 h at 200 μg ml−1. Isotype controls were performed with the isotype-matched murine antibody UPC 10 (IgG2a) as primary antibodies for the same period of time at 200 μg ml−1. Positive staining was defined as a venule displaying brown reaction product.
Isolation of human umbilical vein endothelial cells
Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical cords following standard procedures (Jaffe et al., 1973). Cells were plated on gelatine-coated (0.5 mg ml−1) dishes and cultured in endothelial growth medium-2 (EGM2). Cells from passages 1–3 were used in the experiments. Cytotoxicity of the PDE inhibitors was excluded by measuring lactate dehydrogenase release in culture supernatants.
PMNL adhesion to TNFα-stimulated HUVEC monolayers
Polymorphonuclear leukocytes (PMNL) were isolated from human peripheral venous blood as described (Hatzelmann and Schudt, 2001). HUVEC monolayers were cultured in endothelial cell basal medium with 2% fetal calf serum over 12 h before the experiment. The PMNL adhesion assay followed two different protocols.
Protocol 1: Endothelial cells were stimulated over 3 h with 0.3 ng ml−1 TNFα in endothelial cell basal medium with 2% fetal bovine serum (FBS). Stimulation medium was removed and HUVEC were rinsed in Hanks-buffered saline solution (HBSS) (with Ca2+ and Mg2+). HUVEC were preincubated with roflumilast, roflumilast N-oxide (10 pM–1 μM), rolipram, cilomilast (0.1 nM–10 μM), motapizone (10 μM), adenosine deaminase (ADA) (1 U ml−1) or vehicle (dimethyl sulphoxide, DMSO, 0.2% final concentration) and then PMNL (5 × 104 cells per 500 μl per well) were added to HUVEC at a final volume of 500 μl per well in HBSS.
Protocol 2: Medium was replaced by HBSS and compounds (Protocol 1) were added to non-stimulated HUVEC monolayers followed by PMNL (5 × 105 cells per 500 μl per well) and N-formyl-methionyl-leucyl-phenylalanine (fMLP) (1 μM).
After 30 min, non-adherent PMNL were removed. Adherent cells were quantified, as described before (Schierwagen et al., 1990) by measuring myeloperoxidase (MPO) activity following lysis of PMNL. The number of adherent PMNL per mm2 of HUVEC monolayers was calculated based on a calibration curve. Preliminary experiments showed that 0.3 ng ml−1 TNFα or 1 μM fMLP, as used in the main experiments, produced stable but submaximal PMNL adhesion. None of the compounds affected the MPO assay. Under our conditions, MPO release from activated PMNL was found <5% of total MPO in control experiments.
Quantitation of E-selectin mRNA in HUVEC by real-time reverse-transcription-PCR
HUVEC monolayers were cultured in endothelial basal medium supplemented with 2% FBS overnight and then preincubated with roflumilast N-oxide (1 nM–1 μM), or 10 μM motapizone, or vehicle (DMSO 0.2%) for 15 min followed by stimulation with 30 pg ml−1 TNFα. After 2 h, medium was removed, cells were washed twice with phosphate-buffered saline (PBS) and subsequently lysed (350 μl per well) in buffer RLT supplemented with 1 mM β-mercaptoethanol. RNA was isolated using the RNeasy Mini Kit according to the instructions of the manufacturer. Reverse transcription was performed with 0.5–1 μg RNA using avian myeloblastosis virus (AMV) reverse transcriptase. Quantitative PCR for E-selectin mRNA was performed using the ABI prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. The primer and probe set for E-selectin mRNA was from Applied Biosystems (Assay-on-demand Hs00950401_m1). The primer and probe set for 18sRNA (calibrator) was as described before (Peter et al., 2007).
From the determined CT values the x-fold change compared to a reference was calculated using the 2−ΔΔCT procedure as described by the manufacturer (Applied Biosystems). Initial experiments revealed a submaximal increase of E-selectin mRNA at 30 pg ml−1 TNFα that was selected for the main studies.
Measurement of E-selectin in HUVEC
Confluent HUVEC in 96-well plates were cultured in endothelial cell basal medium with 2% FBS for 12 h. Cells were preincubated with PDE4 inhibitors (1 pM–1 μM roflumilast or roflumilast N-oxide, 10 pM–10 μM rolipram, 100 pM–100 μM cilomilast), or 10 μM motapizone (M), or vehicle (DMSO 0.2%) for 15 min followed by stimulation with 30 pg ml−1 TNFα over 3 h (conditions selected from pilot studies). E-selectin was assessed by cell surface enzyme-linked immunosorbent assay as described (Blease et al., 1998) with modifications. Cells were fixed in 10% neutral-buffered formalin solution, blocked with PBS supplemented with 1% BSA and 1% sheep serum and incubated with the antihuman E-selectin mAb at 1.65 μg ml−1 at 100 μl per well for 30 min. After several washes, secondary antibody (sheep anti mouse IgG coupled to horseradish peroxidase) was added. Peroxidase activity was measured using the 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate system.
CD11b expression on neutrophils
CD11b measurements were adapted as described (Berends et al., 1997) with modifications. Human neutrophil surface CD11b was determined in a whole-blood assay. Duplicate samples (100 μl) of citrated whole blood were incubated with 10 μl of roflumilast, roflumilast N-oxide, rolipram (1 nM–10 μM), or cilomilast (10 nM–100 μM), or vehicle (DMSO 0.1%) for 20 min at 37°C. Then the cells were stimulated with 1 μM fMLP for another 20 min. A saturating amount of an antihuman CD11b-fluorescein isothiocyanate (FITC) mAb (10 μg in 10 μl) was then added for 20 min on ice. Red blood cells were removed with an EPICS Q-PREP system (Coulter Electronics, Hialeah, Florida).
In another set of experiments, 100 μl whole blood samples were incubated for 15 min with or without ADA (1 U ml−1). Roflumilast (10 nM–10 μM) was added for another 20 min and CD11b expression on fMLP stimulated neutrophils was determined as described above.
To determine whether CD11b expression was also reduced after in vivo pretreatment with roflumilast, a blood sample was obtained from rats i.p. injected with saline or LPS with or without roflumilast (10 μmol kg−1) pretreatment. Duplicate samples (100 μl) were incubated with 10 μl of an anti-rat CD11b-FITC mAb for 20 min on ice. CD11b expression on neutrophils was determined as described above.
All analyses were performed with an EPICS XL-MCL flow cytometer (Beckman-Coulter, Hialeah, FL, USA) with a 15 mW Argon Laser tuned at 488 nm as described previously (Sanz et al., 2005b).
In vivo vascular permeability
Male Sprague–Dawley rats were prepared for intravital microscopy and the degree of vascular albumin leakage from mesenteric venules was quantified as described previously (Johnston et al., 1999). Briefly, FITC-labelled bovine albumin (25 mg kg−1) was administered to the rats intravenously at the start of the experiment, and FITC-derived fluorescence (excitation wavelength 450–490 nm; emission wavelength 520 nm) was detected using a charge-coupled device camera model XC-77 (Hamamatsu Photonics, Hamamatsu City, Japan) with a C2400-68 intensifier head (Hamamatsu Photonics) and a C240-60 charge-coupled camera control unit. Image analysis software (analysis 2.11, analysis DOCU) was used to determine the intensity of FITC-albumin-derived fluorescence within the lumen of the venule and in the adjacent perivascular tissue. Background was defined as the fluorescence intensity before FITC-albumin administration. The index of vascular albumin leakage was determined according to the following ratio expressed as a percentage: permeability index=(mean interstitial intensity−background)/(venular intensity−background) × 100%. Roflumilast was given at single oral doses of 0.1–10 μmol kg−1 1 h before histamine. The mesentery of untreated and treated rats was superfused with warmed buffer supplemented with histamine (100 μM). Videorecordings and fluorescence measurements recorded at 1 h after start of histamine superfusion were taken for the analyses.
In vitro macromolecule permeability of HUVEC monolayers
Permeability of HUVEC monolayers for macromolecules was measured as described (Langeler and van Hinsbergh, 1988) with modifications. HUVEC (7.3 × 104 cells per insert) were plated on 3 μm polycarbonate Transwell filters (33 mm2 surface area) pre-coated with 10 μg cm−2 fibronectin and cultured in EGM2 over 4–5 days. Immediately before the experiments medium was replaced by M199 with 1% BSA. HUVEC were preincubated with PDE4 inhibitors (10 pM–1 μM roflumilast, roflumilast N-oxide, rolipram or 100 pM–100 μM cilomilast), or 10 μM motapizone, or vehicle (DMSO 0.2%) for 15 min. Permeability was elicited by thrombin (1 U ml−1) and simultaneously, horseradish peroxidase (5 μg ml−1) was added to the upper wells and the permeation was assessed after 1 h. Peroxidase activity was measured in an aliquot of the lower well using the TMB substrate system.
Statistical analysis
Data are presented as mean±s.e.mean. Statistical analysis of results was carried out by analysis of variance followed by the Bonferroni test or by Student's t-test as appropriate (GraphPad Prism Software Inc., San Diego, CA, USA) with a significance level of α=0.05.
Reagents
The following compounds were purchased from Sigma-Aldrich, St. Louis, MO, USA: fMLP, BSA, LPS (Escherichia coli serotype 0127 :B8), pentobarbital, UPC10 (IgG2a class), histamine, Triton X100, TMB liquid substrate system, neutral-buffered formalin solution, horseradish peroxidase, thrombin, gelatine, sheep serum, FITC-albumin and dextran. ADA was from Sigma-Aldrich or Merck Biosciences, Darmstadt, Germany. Dispase was from Roche Diagnostics GmbH, Mannheim, Germany. Antibodies RMP-1 and RME-1 were generated as described previously (Walter et al., 1997a, 1997b). Biotinylated anti-mouse IgG (H+L) (from goat) and Vectastain ABC Kit were from Vector Laboratories, Burlingame, CA, USA. FITC-conjugated antihuman-CD11b (clone ICRF 44) and phycoerythrin (PE)-conjugated anti-rat-CD11b (clone OX-42) were from Serotec, Madrid, Spain. TNFα, antihuman E-selectin (clone BBIG-E4) were from R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany. Sheep anti-mouse horseradish-conjugated Ig antibody was from Amersham plc, Little Chalfont, UK. EGM2 medium was purchased from PromoCell GmbH, Heidelberg, Germany or Cambrex Bio Science Verviers, S.p.r.l., Verviers, Belgium. Medium 199 (M199), heat-inactivated FBS, PBS and HBSS were from InVitrogen Ltd, Paisley, UK. Buffer RLT, RNeasy Mini Kit, QIAShredder, RNase-free DNase set were all from Qiagen GmbH, Hilden, Germany. AMV reverse transcriptase and random oligonucleotide primers were from Roche Diagnostics, Mannheim, Germany. PCR Master Mix Plus was obtained from Eurogentec SA, Seraing, Belgium and dATP, dCTP, dTTP and dGTP were from Larova GmbH, Teltow, Germany. HTS Transwells-24 (6.5 mm diameter and 3 μm pore size) were from Corning BV, Schiphol-Rijk, The Netherlands. The PDE4 inhibitors roflumilast and its N-oxide (WO9501338), cilomilast (WO9319749) and racemic R, S-rolipram (German Pat. 2413935/3, 1974) were synthesized at the chemical facilities of ALTANA Pharma AG, Konstanz, Germany essentially as described in the corresponding patents. Motapizone was a gift from Rhône-Poulenc Rorer (Köln, Germany; now Sanofi-Aventis).
For in vivo studies, roflumilast was suspended in methocel/PEG400 and administered p.o. by gavage (4 ml kg−1). The control group received methocel/PEG400. For in vitro studies, the final DMSO concentration in the studies 0.2% (v/v), which on its own did not affect endothelial cell functions.
Results
Roflumilast inhibits LPS-induced leukocyte rolling, adhesion and emigration and expression of P- and E-selectin in rat mesenteric venules in vivo
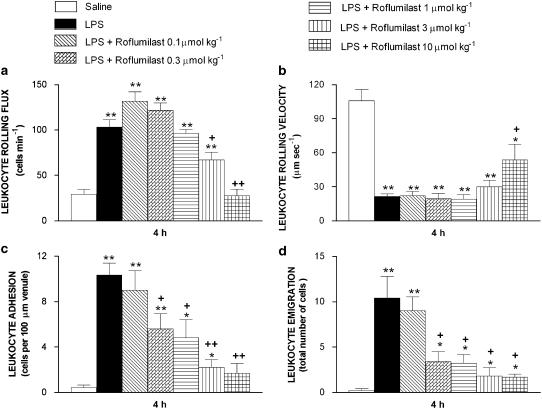
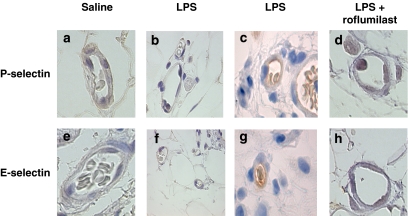
Leukocyte rolling flux, adhesion and emigration were increased, whereas leukocyte rolling velocity (Vwbc) was decreased in rat mesenteric post-capillary venules 4 h after LPS (Figure 1). Roflumilast administered 1 h before LPS at single oral doses of 0.1–10 μmol kg−1 progressively reversed these measures of leukocyte–endothelial interactions. ID50 for inhibition of LPS-induced adhesion and emigration were 0.5 and 0.2 μmol kg−1, respectively. On the other hand, roflumilast was less effective in reducing LPS-induced leukocyte rolling flux (ID50=2.6 μmol kg−1). Inhibition of cell adhesion and emigration by roflumilast was significant at ⩾0.3 μmol kg−1, whereas rolling was significantly reduced at doses ⩾3 μmol kg−1. At the highest dose of roflumilast (10 μmol kg−1), leukocyte rolling flux, adhesion and emigration were suppressed by 100, 87 and 85%, respectively. On the other hand, the reduction by LPS of Vwbc was reversed by only 59% at 10 μmol kg−1 roflumilast and lower doses remained ineffective. The number of circulating leukocytes, MABP and venular wall shear rate remained unaltered (Table 1). Immunohistochemical analysis revealed significant increases in the expression of P- and E-selectin in rat mesenteric microvasculature at 4 h after LPS. Roflumilast (10 μmol kg−1) inhibited the expression of both endothelial adhesion molecules (Figure 2). Fluorescence-activated cell sorter analysis of rat peripheral whole blood showed increased CD11b expression on neutrophils from animals i.p. injected with LPS (4.6±0.4 mean fluorescence intensity (MFI)) compared to that on neutrophils from rats injected with saline (1.8±0.0 MFI; P<0.01; n=4). Roflumilast (10 μmol kg−1) significantly inhibited this LPS-induced expression of this CAM by 47% (n=4).
Figure 1.
Effect of roflumilast on LPS-induced leukocyte rolling flux (a), rolling velocity (b), adhesion (c) and emigration (d) in rat mesenteric postcapillary venules. Parameters were measured 4 h after i.p. injection of 5 ml of saline or 5 ml of LPS (0.2 μg ml−1) in the following experimental groups: untreated rats exposed to buffer (negative control), untreated rats exposed to LPS (positive control) and LPS-exposed rats pretreated with roflumilast (0.1–10 μmol kg−1 p.o., 1 h before LPS injection). Data are mean±s.e.mean from five to six rats per group; *P<0.05 or **P<0.01 compared to negative control; +P<0.05 or ++P<0.01 compared to positive control. LPS, lipopolysaccharide.
Table 1.
Haemodynamic parameters at 4 h following saline or LPS (1 μg per rat i.p.) in the presence or absence of roflumilast (10 μmol kg−1 p.o.)
| Treatment | Leukocyte (cellsμl−1) | MABP (mm Hg) | Shear rate (s−1) |
|---|---|---|---|
| Saline | 3946±865 | 105±7 | 561±43 |
| LPS | 3384±807 | 108±4 | 467±66 |
| LPS+roflumilast | 4076±911 | 111±5 | 516±56 |
Abbreviations: i.p., intraperitoneal; LPS, lipopolysaccharide; MABP, mean arterial blood pressure; p.o, per os.
Values are mean±s.e.mean from five to six rats per group. No significant changes between the different groups were observed.
Figure 2.
Representative photomicrographs of rat mesenteric venules showing immunolocalization of P- and E-selectin expression in animals untreated and pretreated with roflumilast (10 μmol kg−1 p.o.) after LPS exposure. P-selectin expression after 4 h saline (a) or LPS exposure in the untreated group using a primary control antibody (b) or LPS exposure in the untreated group using an anti-rat P-selectin mAb (c) and roflumilast-pretreated group using an anti-rat P-selectin mAb (d). E-selectin expression after 4 h saline (e) or LPS exposure in the untreated group using a primary control antibody (f) or LPS exposure in the untreated group using an anti-rat E-selectin mAb (g) and roflumilast-pretreated group using an anti-rat E-selectin mAb (h). Brown reaction product indicates positive immunoperoxidase localization for all CAMs on the vascular endothelium. All panels are lightly counterstained with hematoxylin/eosin and are of the same magnification (× 400). Results are representative of five to six experiments for each treatment. CAM, cell adhesion molecule; LPS, lipopolysaccharide.
Inhibition of PMNL adherence to HUVEC by roflumilast N-oxide in vitro
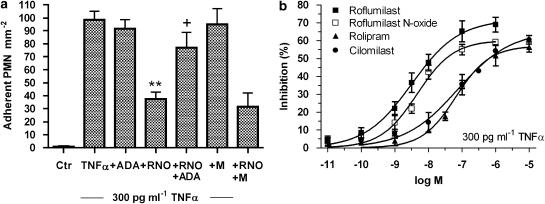
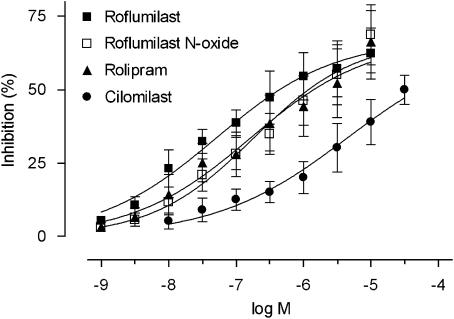
To complement these findings in vivo, we assessed, in vitro, if roflumilast N-oxide directly reduced adhesion of non-stimulated human peripheral blood-derived PMNL to HUVEC activated with 0.3 ng ml−1 TNFα over 3 h (Protocol 1) or adhesion of fMLP-stimulated PMNL to non-stimulated HUVEC (Protocol 2) in vitro. In Protocol 1, complete and selective inhibition of PDE4 by 1 μM roflumilast N-oxide resulted in approximately 64% (P<0.001 versus TNFα) reduction of TNFα-induced PMNL adhesion to HUVEC (Figure 3a). This effect was unchanged in the additional presence of the PDE3 inhibitor motapizone (10 μM); this inhibitor was inactive on its own. ADA significantly reversed the inhibition of PMNL adhesion to TNFα-prestimulated neutrophils (Figure 3a). Roflumilast N-oxide was as potent as roflumilast but more potent than rolipram or cilomilast in decreasing TNFα-elicited PMNL adhesion (Figure 3b; Table 2). In Protocol 2, adhesion of fMLP-activated PMNL to HUVEC was reduced by roflumilast N-oxide and rolipram at IC50 of 1.2 and 47 nM, respectively, with a maximum effect of approximately 60–70% (data not shown).
Figure 3.
Adhesion of PMNL to TNFα-prestimulated HUVEC and its response to PDE inhibitors. Endothelial cell monolayers were stimulated with 0.3 ng ml−1 TNFα for 3 h. Medium was removed and PDE4 inhibitors (roflumilast N-oxide (RNO) or roflumilast (10 pM–1 μM), or rolipram or cilomilast (0.1 nM–10 μM)), or 10 μM motapizone (M), or 1 U ml−1 ADA, followed by PMNL (50 000 cells per well) were added for 30 min. The amount of PMNL adherent to HUVEC monolayers was determined based on measuring MPO activity in lysed PMNL as described in Materials and methods. (a) Comparison of 1 μM RNO, 10 μM M or 1 U ml−1 ADA. The number of PMNL attached to HUVEC per mm2 is shown as mean±s.e.mean from six experiments in triplicates. **P<0.01 versus TNFα, +P<0.05 versus TNFα and RNO (b) Concentration-dependent inhibition by roflumilast N-oxide, roflumilast, cilomilast or rolipram. Results (means±s.e.mean from four experiments, in triplicates) were evaluated as percent inhibition of the TNFα-induced PMNL adhesion. ADA, adenosine deaminase; HUVEC, human umbilical vein endothelial cells; PDE4, phosphodiesterase 4; TNFα, tumour necrosis factor-α.
Table 2.
Potency of PDE4 inhibitors in regulating endothelial cell and neutrophil functions and their interaction in vitro
| Stimulus |
IC50 (nM) |
|||||
|---|---|---|---|---|---|---|
| Motapizone (10 μM) | Roflumilast | Roflumilast N-oxide | Rolipram | Cilomilast | ||
| Adhesion (PMNL/HUVEC) | 0.3 ng ml−1 TNFα | No | 3.2 | 4.5 | 65 | 74.6 |
| 1 μM fMLP | No | 1.2 | 47 | |||
| E-selectin (HUVEC) | 30 pg ml−1 TNFα | Yes | 2.6 | 4.6 | 353 | 936 |
| CD11b (neutrophils) | 1 μM fMLP | No | 0.5 | 6.2 | 35 | 265 |
| Permeability (HUVEC) | 1 U ml−1 thrombin | No | 3.3 | 1.7 | 56.4 | 207 |
| Yes | 0.7 | 0.6 | 27.8 | 83.4 | ||
| PDE4 inhibition (PMNL)a | — | — | 0.8 | 2 | 210 | 120 |
Abbreviations: fMLP, N-formyl-methionyl-leucyl-phenylalanine; HUVEC, human umbilical vein endothelial cells; PDE4, phosphodiesterase 4; PMNL, polymorphonuclear leukocytes; TNFα, tumour necrosis factor-α.
IC50 values were calculated from concentration–inhibition curves (see figures) by nonlinear regression using GraphPad Prism software, except for the values shown for CD11b expression. Here, the IC50 values from the data in Figure 6 have been corrected for the different extent of binding to plasma proteins for each PDE4 inhibitor (see Discussion).
IC50 values for inhibition of PDE4 from human neutrophils measured at 0.5 μM cAMP substrate concentration were taken from Hatzelmann and Schudt (2001).
Roflumilast N-oxide inhibits TNFα-induced E-selectin on HUVEC in vitro
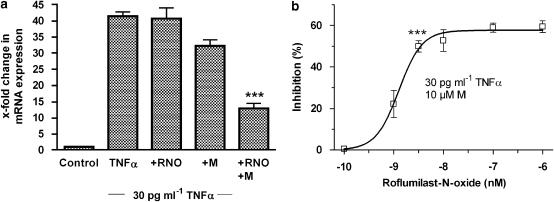
First, effects of the PDE4 inhibitor on E-selectin mRNA expression were analysed. TNFα (30 pg ml−1) enhanced E-selectin transcripts by ∼40-fold and this enhancement was not affected by 1 μM roflumilast N-oxide alone (Figure 4a). However, the PDE4 inhibitor significantly reduced E-selectin mRNA by another ∼60% in the presence of motapizone (10 μM). Motapizone at this concentration decreased E-selectin expression by ∼20%, when used alone (Figure 4a). Inhibition of E-selectin mRNA expression by this combination of PDE inhibitors was dependent on the concentration of roflumilast N-oxide and a significant reduction of approximately 50% was achieved at 3 nM roflumilast N-oxide (Figure 4b).
Figure 4.
Relative quantitation of E-selectin mRNA in HUVEC stimulated with TNFα in the presence or absence of PDE inhibitors. HUVEC were preincubated with roflumilast N-oxide (RNO, 0.1 nM–1 μM), or 10 μM motapizone (M), for 15 min and stimulated with 30 pg ml−1 TNFα over 2 h. E-selectin mRNA was evaluated by real-time RT-PCR as detailed in the Materials and methods. The change in mRNA expression compared to control (defined as 1) was calculated from measured CT values obtained for E-selectin relative to 18S mRNA with the 2−ΔΔCT procedure. Results are shown as the means±s.e.mean from three experiments in duplicate. ***P<0.001 relative to TNFα+M. (a) Effects of 1 μM RNO in the presence or absence of 10 μM motapizone. Columns show the x-fold change in mRNA expression versus control; (b) E-selectin mRNA was concentration-dependently reduced by RNO in the presence of a fixed concentration of motapizone (10 μM). The percent inhibition of the increase in mRNA expression, induced by 30 pg ml−1 TNFα in the presence of 10 μM motapizone is shown. HUVEC, human umbilical vein endothelial cells; RT-PCR, reverse-transcription PCR; TNFα, tumour necrosis factor-α.
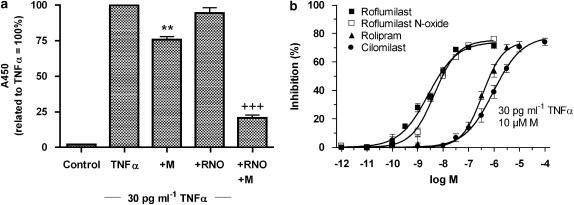
We then measured E-selectin protein (Figure 5) and found that 1 μM roflumilast N-oxide inhibited TNFα-induced E-selectin protein in the presence of the PDE3 inhibitor motapizone (10 μM), in an overadditive manner, but was ineffective alone (Figure 5a). Roflumilast and roflumilast N-oxide were more potent than rolipram and cilomilast in suppressing the expression of E-selectin protein elicited by TNFα in the presence of 10 μM motapizone (Figure 5b).
Figure 5.
Influence of PDE inhibitors on TNFα-induced E-selectin protein on HUVEC. Monolayers of endothelial cells were preincubated with PDE4 inhibitors (1 pM–1 μM roflumilast, roflumilast N-oxide (RNO), rolipram, 100 pM–100 μM cilomilast) or 10 μM motapizone (M) and E-selectin expression was induced following 30 pg ml−1 TNFα over 3 h. E-selectin protein was determined by cell-surface ELISA. (a) Effects of 1 μM RNO, 10 μM motapizone (M) or their combination. Data are related to the absorbance with TNFα alone defined as 100% for each individual experiment. Results are shown from eight experiments in triplicates. **P<0.01 versus TNFα, +++P<0.001 versus TNFα+M, (b) PDE4 inhibitors concentration-dependently decrease E-selectin expression in the presence of 10 μM motapizone (M). Means±s.e.mean of percent inhibition of the absorbance with TNFα in the presence of motapizone are shown. n=3–6 experiments in triplicate. ELISA, enzyme-linked immunosorbent assay; HUVEC, human umbilical vein endothelial cells; PDE4, phosphodiesterase 4; TNFα, tumour necrosis factor-α.
Roflumilast N-oxide inhibits surface CD11b expression on human neutrophils in vitro
The effects of PDE4 inhibitors on fMLP-triggered neutrophil surface CD11b expression were explored in a whole-blood assay where plasma protein binding of the investigated compounds had to be considered. Roflumilast (IC50=51 nM), roflumilast N-oxide (IC50=182 nM) and rolipram (IC50=163 nM) reversed fMLP-induced surface CD11b expression on neutrophils with rather comparable potency, which was higher than that achieved with cilomilast (IC50=4.4 μM; Figure 6). The PDE4 inhibitors were similarly equi-effective affording a maximum inhibition of 65–75%.
Figure 6.
PDE4 inhibitors modulated CD11b expression on human neutrophils. Whole-blood samples were incubated with vehicle, roflumilast roflumilast-N-oxide, rolipram (1 nM–10 μM) or cilomilast (0.01–100 μM) for 20 min at 37°C and then stimulated with 1 μM fMLP for another 20 min. Thereafter, samples were incubated with FITC-conjugated anti CD11b antibody. FITC-fluorescence in the neutrophil gate was quantitated by flow cytometry. Results are presented as percentage inhibition of fMLP-induced CD11b upregulation based on MFI recordings. Data are means±s.e.mean from four to six experiments. FITC, fluorescein isothiocyanate; fMLP, N-formyl-methionyl-leucyl-phenylalanine; MFI, mean fluorescence intensity; PDE4, phosphodiesterase 4.
Adenosine released from fMLP-stimulated neutrophils may act as an autocrine mediator to support the inhibition of neutrophil functions by PDE4 inhibitors. In the presence of ADA (1 U ml−1), the potency of roflumilast to reduce surface CD11b expression on neutrophils decreased by approximately 6.5-fold (IC50=248 nM in the presence of ADA compared to IC50=38 nM in its absence) (data not shown). Adding ADA did not change the maximum effect of PDE4 inhibitors or the extent of fMLP-induced surface CD11b presented on neutrophils.
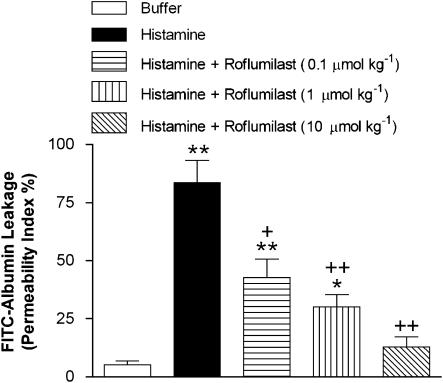
Inhibition of histamine-induced rat mesenteric microvascular permeability by roflumilast in vivo
When histamine was superfused for 1 h on the mesentery of saline-treated rats, plasma protein leakage as reflected by extravasation of FITC-conjugated albumin was increased. Roflumilast (0.1–10 μmol kg−1 p.o., single dose) significantly and dose-dependently reduced FITC-albumin extravasation (Figure 7) with an ID50 of 88 nmol kg−1. At the highest dose used (10 μ mol kg−1), roflumilast reversed histamine-induced extravasation of FITC-albumin by approximately 85%.
Figure 7.
Effects of roflumilast on histamine-induced microvascular permeability in rat mesenteric postcapillary venules in vivo. Microvascular permeability index (see Materials and methods section for definition) was measured 1 h after mesenteric superfusion with 100 μM histamine in the following experimental groups: untreated rats exposed to buffer (negative control); untreated rats exposed to histamine (positive control) and histamine-exposed rats pretreated with roflumilast (0.1–10 μmol kg−1 p.o. 1 h before histamine superfusion). Results are shown as means±s.e.mean from three rats per group; **P<0.01 compared to negative control; ++P<0.01 compared to positive control.
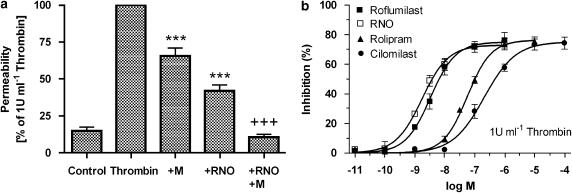
Roflumilast N-oxide reduced thrombin-induced macromolecule permeability in HUVEC monolayers in vitro
Thrombin-induced macromolecule permeability was reduced following complete and selective inhibition of PDE4 (1 μM roflumilast N-oxide) or PDE3 (10 μM motapizone) by ∼65 and 35%, respectively, and was suppressed below baseline by dual-selective inhibition of PDE3 and PDE4 (Figure 8a). Among PDE4 inhibitors, the potencies of roflumilast (IC50=3.3 nM) and roflumilast N-oxide (IC50=1.7 nM) to restore HUVEC barrier integrity impaired by thrombin were very close. However, these potencies were higher than those of rolipram (IC50=56 nM) and cilomilast (IC50=207 nM) (Figure 8b). In the presence of the PDE3 inhibitor motapizone (10 μM), the potency of the PDE4 inhibitors in reducing macromolecule permeation increased by approximately two- to fourfold (Table 2).
Figure 8.
Thrombin-induced macromolecule permeability of HUVEC monolayers was regulated by PDE inhibitors. HUVEC cultured on polycarbonate filters in Transwells were preincubated with PDE4 inhibitors (10 pM–1 μM roflumilast, roflumilast N-oxide (RNO), 100 pM–10 μM rolipram, 100 pM–100 μM cilomilast), or 10 μM motapizone (M), and stimulated with 1 U ml−1 thrombin. Horseradish peroxidase (5 μg ml−1) was added to the upper wells and permeation of the marker protein into the lower wells was assessed after 60 min. (a) Effects of 1 μM roflumilast N-oxide, or 10 μM motapizone. Results are related to macromolecule permeation with 1 U ml−1 thrombin defined as 100% for each individual experiment and given as means±s.e.mean from five experiments in triplicates. ***P<0.001 compared to thrombin alone; +++P<0.001 compared to thrombin+M, (b) PDE4 inhibitors concentration-dependently reduced thrombin-induced macromolecule permeability of HUVEC monolayers. Data from four experiments in triplicate are summarized as means±s.e.mean of percent inhibition of permeability in the presence of thrombin alone. HUVEC, human umbilical vein endothelial cells; PDE4, phosphodiesterase 4.
Discussion and conclusions
The PDE4 inhibitor roflumilast dose-dependently reduced LPS-induced leukocyte–endothelial interactions in rat mesenteric postcapillary venules in a 4-h protocol. In addition, roflumilast suppressed histamine-induced permeability in rat mesenteric microvasculature. Complementary in vitro studies showed that roflumilast N-oxide directly reduced PMNL adherence to HUVEC, neutrophil surface CD11b expression, HUVEC E-selectin expression and macromolecule permeability. Thus, roflumilast decreased endothelial cell activation in vivo and in vitro.
Roflumilast at 10 μmol kg−1 almost completely suppressed LPS-induced leukocyte–endothelial cell interactions in vivo. LPS-induced leukocyte adhesion and emigration were potently reversed by roflumilast. Indeed, extrapolations from pharmacokinetic investigations with roflumilast in Sprague–Dawley rats (data not shown) indicate that at the calculated ID50 values for inhibition of leukocyte adhesion and emigration by the PDE4 inhibitor, free plasma concentrations of roflumilast and roflumilast N-oxide may approach 2–8 nM over the 4-h experimental period corresponding to 50–80% inhibition of PDE4 (Hatzelmann and Schudt, 2001). Therefore, the potency of roflumilast to reduce LPS-induced leukocyte adhesion and emigration in vivo paralleled its capacity to inhibit PDE4. As firm adhesion is mainly governed by leukocyte β2-integrins, it is likely that the potent inhibition of neutrophil surface CD11b upregulation by roflumilast N-oxide in vitro contributed to the strong reduction of LPS-induced leukocyte adhesion in this in vivo model. In fact, in animals pretreated with roflumilast at 10 μmol kg−1, LPS-induced increase of CD11b neutrophil expression was reduced by 47%. On the other hand, leukocyte rolling is governed by a number of CAMs, such as P-/E-selectin, α4-integrin and L-selectin (Johnston et al., 1997; Ley et al., 1998), which may be differentially affected by PDE4 inhibitors. While endothelial P-/E-selectin or neutrophil α4-integrin are reduced (Blease et al., 1998; Sanz et al., 2002; Sullivan et al., 2004 and this study), neutrophil L-selectin may be augmented (Berends et al., 1997). These findings, together with the observation on the complete inhibition of LPS-induced rolling by either L-selectin or α4-integrin antibodies (in the absence of P-selectin) (Johnston et al., 1997), may explain the reduced potency but unchanged efficacy of roflumilast to reduce LPS-induced leukocyte rolling.
Strikingly, roflumilast diminished LPS-induced plasma TNFα increase in Sprague–Dawley rats with approximately the same potency (Bundschuh et al., 2001) as observed for inhibition of LPS-induced leukocyte adhesion and emigration in the current study. Therefore, part of the effects displayed by roflumilast may be due to its rapid inhibition of LPS-induced increase of plasma TNFα in vivo.
In rodents, PDE4 inhibitors may stimulate the hypothalamic–pituitary–adrenal axis. Thus, an increase in endogenous corticosterone plasma levels may, in part, account for the anti-inflammatory effects of PDE4 inhibitors. In mice, plasma corticosterone rose by approximately four- to sixfold after 30 min rolipram administration. The reduction of the LPS-induced TNFα release in an ex vivo whole-blood assay or the ovalbumin-induced pulmonary eosinophilic infiltration by the PDE4 inhibitor was partially reversed by a glucocorticoid receptor antagonist (Pettipher et al., 1997; Kung et al., 2000). In rats, rolipram rapidly (20 min after i.p. injection) and dose-dependently augmented serum corticosterone levels (Kumari et al., 1997). It is therefore possible that the reduction of LPS-induced leukocyte–endothelial cell interactions in the mesenteric postcapillary venules by roflumilast may be attributed, in part, to an increase of serum corticosterone levels. Notwithstanding that, roflumilast and roflumilast N-oxide directly reduced adherence of PMNL to activated HUVEC in vitro.
Given that PDE4 inhibitors attenuate fMLP-, leukotriene B4- or platelet-activating factor-stimulated upregulation of PMNL surface CD11b (Derian et al., 1995; Sato et al., 2002; Meliton et al., 2006 and this study) and considering that endothelial cells activated by cytokines upregulate surface β2-integrin on neutrophils (Kuijpers et al., 1991; Simon et al., 2000), it is proposed that roflumilast N-oxide prevented adherence of resting PMNL to activated endothelial cells by inhibiting the upregulation of PMNL surface β2-integrin. In agreement with this, roflumilast N-oxide or rolipram reduced fMLP-induced PMNL adherence to resting HUVEC with comparable potency and efficacy (Jones et al., 2005 and this study). ADA reversed the inhibition of PMNL adherence to TNFα-activated HUVEC by roflumilast N-oxide and compromised the potency of the PDE4 inhibitor to reduce fMLP-induced surface CD11b on human neutrophils in agreement with earlier studies (Derian et al., 1995; Wollner et al., 1993). Endothelial cells and neutrophils by themselves are strong producers of adenosine that may reinforce the effects of PDE4 inhibitors (Sullivan et al., 2001). These observations suggest that the capacity of roflumilast N-oxide to efficiently reduce neutrophil β2-integrin expression or adherence to endothelial cells may be confined to areas of (neutrophilic) inflammation where adenosine concentrations are reported to be high (10–100 μM) (Hasko and Cronstein, 2004). In contrast, in non-inflamed areas where local adenosine concentrations are low (<1 μM) (Hasko and Cronstein, 2004), the PDE4 inhibitor may be less potent.
Neither roflumilast nor roflumilast N-oxide (up to 1 μM) affected baseline adherence of resting PMNL to unstimulated HUVEC (data not shown). It was shown recently that incubation of HUVEC with roflumilast over 24 h augments baseline IL-8 release, indicating that the PDE4 inhibitor may activate HUVEC. However, this increased IL-8 release was not observed with roflumilast up to 1 μM (that is at concentrations selectively inhibiting PDE4) but occurred at 10 and 100 μM of the compound (McCluskie et al., 2006). At these high concentrations (that is 5000-and 50 000-fold higher than therapeutic plasma levels of roflumilast and roflumilast N-oxide in humans; Bethke et al., 2007), the compound loses its selectivity as an inhibitor of PDE4.
In agreement with previous findings (Pober et al., 1993; Morandini et al., 1996; Blease et al., 1998), dual-selective inhibition of PDE4 and PDE3 reduced HUVEC E-selectin expression by approximately 80%. Motapizone (10 μM), with only approximately 20% inhibition by itself, was synergistic in combination with 1 μM roflumilast N-oxide, where the latter did not affect E-selectin mRNA or protein on its own, reflecting the co-expression of PDE3 and PDE4 in HUVEC (Seybold et al., 2005).
In inflammation, a myriad of mediators directly or indirectly foster endothelial permeability and consequently extravasation of fluids and protein into the extravascular compartment. It is well known that cAMP protects the integrity of the endothelial barrier that is impaired in the presence of thrombin. Recent studies have shown that both protein kinase A and the ‘exchange protein directly activated by cAMP' (Epac) are critical components in the reduction of endothelial permeability by cAMP (Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005; Birukova et al., 2007). Epac-1/Rap1 but also protein kinase A activate Rac, crucial to the provision of an array of cytoskeletal effectors, finally resulting in an improved endothelial barrier. Other mechanisms such as protein kinase A-dependent myosin light chain kinase (MLCK) phosphorylation and RhoA inactivation are also involved in cAMP-dependent protection of the endothelial barrier (Birukova et al., 2007). Consequently, previous investigations have shown that the PDE4 inhibitors rolipram or piclamilast reduce histamine-induced microvascular leakage in guinea pig airways (Ortiz et al., 1993; Raeburn et al., 1994). In our study, roflumilast efficiently suppressed histamine-induced rat mesenteric microvascular permeability in vivo. In fact, among all the in vivo functions explored in this study, microvascular permeability exhibited the highest sensitivity for inhibition by roflumilast. In vitro, roflumilast N-oxide potently diminished thrombin-induced endothelial permeability. The ability of PDE4 and PDE3 inhibitors to protect endothelial barrier integrity in vitro is broadly corroborated by previous studies (Suttorp et al., 1993, 1996; Draijer et al., 1995). It is possible that promoting endothelial barrier integrity by PDE4 inhibitors may potentially contribute to reducing airway oedema in asthma, alveolar oedema in acute lung injury or to mitigating vascular remodelling.
The potencies (IC50) of roflumilast N-oxide or roflumilast compared to rolipram or cilomilast to reduce adherence of PMNL to HUVEC, neutrophil surface CD11b, E-selectin expression and HUVEC permeability are summarized in Table 2 and for comparison, the IC50 for inhibition of PDE4 catalytic activity from human neutrophil extracts (Hatzelmann and Schudt, 2001) are given. For CD11b, IC50 values in the absence of plasma proteins, as shown in Table 2, were estimated from those obtained in the whole-blood assay, considering the following fractions unbound to human plasma: roflumilast 1.1%, roflumilast N-oxide 3.4% (Hauns et al., 2006), rolipram 22%, cilomilast 6% (Hatzelmann and Schudt, 2001). Roflumilast N-oxide and roflumilast reduced endothelial cell and neutrophil functions with IC50 ∼0.5–6.2 nM, which is comparable to their previously reported potency to inhibit PDE4 as well as inflammatory cell functions (Hatzelmann and Schudt, 2001). In addition, plasma concentrations of roflumilast and roflumilast N-oxide required for inhibition of leukocyte adhesion or endothelial permeability by roflumilast in rats in vivo (estimated from ID50) were in the same range as those inhibiting the corresponding endothelial and neutrophil functions in vitro. Roflumilast and roflumilast N-oxide were more potent than rolipram and cilomilast in affecting the investigated endothelial cell functions and endothelial-PMNL adhesion. Cilomilast was the least potent of the four PDE4 inhibitors tested in our assays. This illustrates the higher capacity of roflumilast and its active metabolite to reduce PDE4 activity over rolipram or cilomilast.
In conclusion, by extending earlier investigations that characterized the anti-inflammatory potential of roflumilast, the current study has revealed the capacity of this PDE4 inhibitor to potently suppress the leukocyte–endothelial cell interactions and the increased endothelial permeability that are hallmarks of chronic inflammation in vivo and in vitro.
Acknowledgments
This work was supported by Grants SAF2005-00669 (JC), SAF2005-01649 (MJS) and SAF2003-07206-C02-01 and SAF2006-01002 (EJM) from CICYT (Ministry of Science and Technology, Spanish Government) and research aids 03/166, 03/116, GV04B72, and GV-2004-B-229 from Regional Government (Generalitat Valenciana) and by ALTANA Pharma AG, a member of the Nycomed group, Konstanz, Germany. MAT was supported by grants from Spanish Ministry of Foreign Affairs. ACI was supported by grant MT-7684 from the Canadian Institutes of Health Research.
We thank Dr Angela Schilling and Dr Tanja Henrichs (Medical Writing, ALTANA Pharma AG, Konstanz, Germany) for helpful suggestions during the preparation of this article.
Abbreviations
- ADA
adenosine deaminase
- CAM
cell adhesion molecule
- COPD
chronic obstructive pulmonary disease
- DMSO
dimethyl sulphoxide
- EGM2
endothelial growth medium-2
- FITC
fluorescein isothiocyanate
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- HBSS
Hanks-buffered saline solution
- HUVEC
human umbilical vein endothelial cells
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MABP
mean arterial blood pressure
- MFI
mean fluorescence intensity
- MPO
myeloperoxidase
- PMNL
polymorphonuclear leukocytes
- TMB, 3,3′,5
5′-tetramethylbenzidine
- TNFα
tumour necrosis factor-α
- Vrbc
centerline red blood cell velocity
- Vwbc
leukocyte rolling velocity
Conflict of interest
This research was supported in part by ALTANA Pharma AG, a member of the Nycomed group, Konstanz, Germany.
References
- Banner KH, Trevethick MA. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci. 2004;25:430–436. doi: 10.1016/j.tips.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Berends C, Dijkhuizen B, de Monchy JG, Dubois AE, Gerritsen J, Kauffman HF. Inhibition of PAF-induced expression of CD11b and shedding of L-selectin on human neutrophils and eosinophils by the type IV selective PDE inhibitor, rolipram. Eur Respir J. 1997;10:1000–1007. doi: 10.1183/09031936.97.10051000. [DOI] [PubMed] [Google Scholar]
- Bethke TD, Bohmer GM, Hermann R, Hauns B, Fux R, Morike K, et al. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47:26–36. doi: 10.1177/0091270006294529. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson J, et al. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease K, Burke-Gaffney A, Hellewell PG. Modulation of cell adhesion molecule expression and function on human lung microvascular endothelial cells by inhibition of phosphodiesterases 3 and 4. Br J Pharmacol. 1998;124:229–237. doi: 10.1038/sj.bjp.0701833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell-Smith V, Page CP. Roflumilast: a phosphodiesterase-4 inhibitor for the treatment of respiratory disease. Expert Opin Investig Drugs. 2006;15:1105–1113. doi: 10.1517/13543784.15.9.1105. [DOI] [PubMed] [Google Scholar]
- Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297:280–290. [PubMed] [Google Scholar]
- Burgess JK, Oliver BG, Poniris MH, Ge Q, Boustany S, Cox N, et al. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. J Allergy Clin Immunol. 2006;118:649–657. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahishi J, Luscinska FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-mediated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol. 1995;154:308–317. [PubMed] [Google Scholar]
- Draijer R, Atsma DE, van der LA, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res. 1995;76:199–208. doi: 10.1161/01.res.76.2.199. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takahura N, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell–cell contact to enhance endothelial barrier function through an Epac-Rap1 signalling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:326–333. doi: 10.1513/pats.200504-041SR. [DOI] [PubMed] [Google Scholar]
- Growcott EJ, Spink KG, Ren X, Afzal S, Banner KH, Wharton J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir Res. 2006;7:9. doi: 10.1186/1465-9921-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NR, Russell JM, Granger DN. Mediators of endotoxin-induced leukocyte adhesion in mesenteric postcapillary venules. Circ Shock. 1994;43:155–160. [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–279. [PubMed] [Google Scholar]
- Hauns B, Hermann R, Hunnemeyer A, Herzog R, Hauschke D, Zech K, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46:1146–1153. doi: 10.1177/0091270006291621. [DOI] [PubMed] [Google Scholar]
- House SD, Lipowsky HH. Leukocyte–endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B, Gaboury JP, Suematsu M, Kubes P. Nitric oxide inhibits microvascular protein leakage induced by leukocyte adhesion-independent and adhesion-dependent inflammatory mediators. Microcirculation. 1999;6:153–162. [PubMed] [Google Scholar]
- Johnston B, Walter UM, Issekutz AC, Issekutz TB, Anderson DC, Kubes P. Differential roles of selectins and the alpha4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J Immunol. 1997;159:4514–4523. [PubMed] [Google Scholar]
- Jones NA, Boswell-Smith V, Lever R, Page CP. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18:93–101. doi: 10.1016/j.pupt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kooistra MRH, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Kubes P, Kerfoot SM. Leukocyte recruitment in the microcirculation: the rolling paradigm revisited. News Physiol Sci. 2001;16:76–80. doi: 10.1152/physiologyonline.2001.16.2.76. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Hakkert BC, Hoogerwerf M, Leeuwenberg JF, Roos D. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol. 1991;147:1369–1376. [PubMed] [Google Scholar]
- Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003;307:349–355. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- Kumari M, Cover PO, Poyser RH, Buckingham JC. Stimulation of the hypothalamic–pituitary–adrenal axis in the rat by three selective type-4 phosphodiesterase inhibitors: in vitro and in vivo studies. Br J Pharmacol. 1997;121:459–468. doi: 10.1038/sj.bjp.0701158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung TT, Crawley Y, Luo B, Young S, Kreutner W, Chapman RW. Inhibition of pulmonary eosinophilia and airway hyperresponsiveness in allergic mice by rolipram: involvement of endogenously released corticosterone and catecholamines. Br J Pharmacol. 2000;130:457–463. doi: 10.1038/sj.bjp.0703308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeler EG, van Hinsbergh VW. Characterization of an in vitro model to study the permeability of human arterial endothelial cell monolayers. Thromb Haemost. 1988;60:240–246. [PubMed] [Google Scholar]
- Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172:848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- Mata M, Sarria B, Buenestado A, Cortijo J, Cerda M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005;60:144–152. doi: 10.1136/thx.2004.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie K, Klein U, Linnevers C, Ji Y, Yang A, Husfeld C, et al. Phosphodiesterase type 4 inhibitors cause proinflammatory effects in vivo. J Pharmacol Exp Ther. 2006;319:468–476. doi: 10.1124/jpet.106.105080. [DOI] [PubMed] [Google Scholar]
- Meliton AY, Munoz NM, Lambertino A, Boetticher E, Learoyd J, Zhu X, et al. Phosphodiesterase 4 inhibition of beta2-integrin adhesion caused by leukotriene B4 and TNF-alpha in human neutrophils. Eur Respir J. 2006;28:920–928. doi: 10.1183/09031936.06.00028406. [DOI] [PubMed] [Google Scholar]
- Morandini R, Ghanem G, Portier-Lemarie A, Robaye B, Renaud A, Boeynaems JM. Action of cAMP on expression and release of adhesion molecules in human endothelial cells. Am J Physiol. 1996;270:H807–H816. doi: 10.1152/ajpheart.1996.270.3.H807. [DOI] [PubMed] [Google Scholar]
- Ortiz JL, Cortijo J, Valles JM, Bou J, Morcillo EJ. Rolipram inhibits airway microvascular leakage induced by platelet-activating factor, histamine and bradykinin in guinea-pigs. J Pharm Pharmacol. 1993;45:1090–1092. doi: 10.1111/j.2042-7158.1993.tb07188.x. [DOI] [PubMed] [Google Scholar]
- Peter D, Jin SLC, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178:4820–4831. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- Pettipher ER, Eskra JD, Labasi JM. The inhibitory effect of rolipram on TNF-α production in mouse blood ex vivo is dependent upon the release of corticosterone and adrenaline. Cytokine. 1997;9:582–586. doi: 10.1006/cyto.1997.0205. [DOI] [PubMed] [Google Scholar]
- Pober JS, Slowik MR, De Luca LG, Ritchie AJ. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993;150:5114–5123. [PubMed] [Google Scholar]
- Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroeker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- Raeburn D, Underwood SL, Lewis SA, Woodman VR, Battram CH, Tomkinson A, et al. Anti-inflammatory and bronchodilator properties of RP 73401, a novel and selective phosphodiesterase type IV inhibitor. Br J Pharmacol. 1994;113:1423–1431. doi: 10.1111/j.1476-5381.1994.tb17156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Williams RO, Mason LJ, Mauri C, Marinova-Mutafchieva L, Malfait AM, et al. Suppression of TNF-alpha expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- Sanz MJ, Alvarez A, Piqueras L, Cerda M, Issekutz AC, Lobb RR, et al. Rolipram inhibits leukocyte-endothelial cell interactions in vivo through P- and E-selectin downregulation. Br J Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005a;106:269–297. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Nabah YN, Cerda-Nicolas M, O'Connor JE, Issekutz AC, Cortijo J, et al. Erythromycin exerts in vivo anti-inflammatory activity downregulating cell adhesion molecule expression. Br J Pharmacol. 2005b;144:190–201. doi: 10.1038/sj.bjp.0706021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Sato S, Yamamoto T, Ishikawa S, Onizuka M, Sakakibara Y. Phosphodiesterase type 4 inhibitor reduces the retention of polymorphonuclear leukocytes in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1376–L1381. doi: 10.1152/ajplung.00433.2001. [DOI] [PubMed] [Google Scholar]
- Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, et al. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood. 2005;105:3569–3576. doi: 10.1182/blood-2004-07-2729. [DOI] [PubMed] [Google Scholar]
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- Sommer N, Loschmann PA, Northoff GH, Weller M, Steinbrecher A, Steinbach JP, et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995;1:244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, et al. Activation of A2A adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br J Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N, Ehreiser P, Hippenstiel S, Fuhrmann M, Krull M, Tenor H, et al. Hyperpermeability of pulmonary endothelial monolayer: protective role of phosphodiesterase isoenzymes 3 and 4. Lung. 1996;174:181–194. doi: 10.1007/BF00173310. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest. 1993;91:1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola AM. PDE4 inhibitors in COPD—a more selective approach to treatment. Respir Med. 2004;98:495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Walter UM, Ayer LM, Manning AM, Frenette PS, Wagner DD, Hynes RO, et al. Generation and characterization of a novel adhesion function blocking monoclonal antibody recognizing both rat and mouse E-selectin. Hybridoma. 1997a;16:355–361. doi: 10.1089/hyb.1997.16.355. [DOI] [PubMed] [Google Scholar]
- Walter UM, Ayer LM, Wolitzky BA, Wagner DD, Hynes RO, Manning AM, et al. Characterization of a novel adhesion function blocking monoclonal antibody to rat/mouse P-selectin generated in the P-selectin-deficient mouse. Hybridoma. 1997b;16:249–257. doi: 10.1089/hyb.1997.16.249. [DOI] [PubMed] [Google Scholar]
- Wollin L, Barsig J, Marx D, Wohlsen A, Beume R. Inhibition by roflumilast versus cilomilast of pulmonary leukocyte accumulation and TNFα release in a rat model of LPS-induced pulmonary neutrophilia. Eur Respir J. 2003;22:107s. [Google Scholar]
- Wollin L, Bundschuh DS, Wohlsen A, Marx D, Beume R. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther. 2006;19:343–352. doi: 10.1016/j.pupt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wollner A, Wollner S, Smith JB. Acting via A2 receptors, adenosine inhibits the upregulation of Mac-1 (Cd11b/CD18) expression on FMLP-stimulated neutrophils. Am J Respir Cell Mol Biol. 1993;9:179–185. doi: 10.1165/ajrcmb/9.2.179. [DOI] [PubMed] [Google Scholar]