Abstract
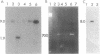
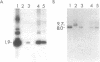
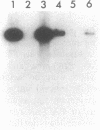
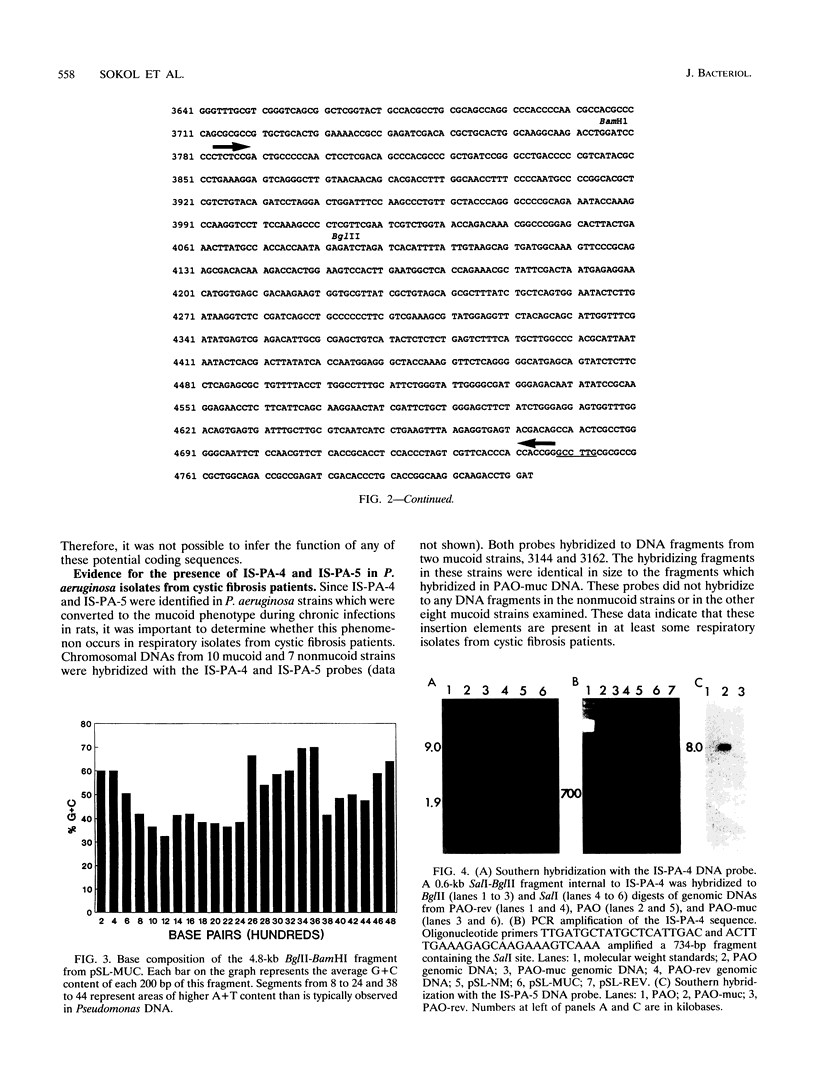
The conversion of Pseudomonas aeruginosa PAO to the mucoid phenotype has been reported for a chronic pulmonary infection model in rats (D. E. Woods, P. A. Sokol, L. E. Bryan, D. G. Storey, S. J. Mattingly, H. J. Vogel, and H. Ceri, J. Infect. Dis. 163:143-149, 1991). This conversion was associated with a genetic rearrangement upstream of the exotoxin A gene. To characterize the genetic rearrangement, the region upstream of the toxA gene was cloned from PAO, PAO-muc (a mucoid strain), and PAO-rev (a nonmucoid revertant strain). The nucleotide sequence of a 4.8-kb fragment from PAO-muc was determined. A+T-rich regions of approximately 2 kb (IS-PA-4) and 0.4 kb (IS-PA-5) were identified in this fragment. DNA probes constructed internal to these regions hybridized to PAO-muc but not to PAO or PAO-rev, suggesting that PAO-muc contains an insertion element. Sequence analysis of the nonmucoid clones indicated that a 2,561-bp fragment corresponding to IS-PA-4 and a 992-bp fragment corresponding to IS-PA-5 were not present in PAO or PAO-rev. Both nonmucoid clones, however, contained in the same location as IS-PA-4, a 1,313-bp region which was not present in PAO-muc. DNA probes complementary to this sequence, designated IS-PA-6, did not hybridize with PAO-muc, indicating that this sequence had been replaced upon conversion to the mucoid phenotype. Between IS-PA-4 and IS-PA-5 there was a 500-bp sequence which was 94% identical to the 500-bp sequence downstream of IS-PA-6. These insertion elements had some DNA sequence similarity to plasmid and transposon sequences, suggesting that they may be of plasmid origin. IS-PA-4 and IS-PA-5 were shown also to be present in two mucoid isolates from cystic fibrosis patients. The insertions occurred in the same location upstream of the toxA gene, suggesting that this type of genetic recombination may also be associated with mucoid conversion in some P. aeruginosa clinical isolates.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett D. H., Silverman M. Nucleotide sequence of IS492, a novel insertion sequence causing variation in extracellular polysaccharide production in the marine bacterium Pseudomonas atlantica. J Bacteriol. 1989 Mar;171(3):1763–1766. doi: 10.1128/jb.171.3.1763-1766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette L., Roy P. H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992 Feb;174(4):1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Winnie J. N., Fritzinger D., Pridmore R. D. The nucleotide sequence of the tnpA gene completes the sequence of the Pseudomonas transposon Tn501. Nucleic Acids Res. 1985 Aug 12;13(15):5657–5669. doi: 10.1093/nar/13.15.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M., Hadero A., Crawford I. P. Sequence of the Pseudomonas aeruginosa trpI activator gene and relatedness of trpI to other procaryotic regulatory genes. J Bacteriol. 1989 Jan;171(1):172–183. doi: 10.1128/jb.171.1.172-183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comanducci M., Ricci S., Ratti G. The structure of a plasmid of Chlamydia trachomatis believed to be required for growth within mammalian cells. Mol Microbiol. 1988 Jul;2(4):531–538. doi: 10.1111/j.1365-2958.1988.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Deretic V., Dikshit R., Konyecsni W. M., Chakrabarty A. M., Misra T. K. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol. 1989 Mar;171(3):1278–1283. doi: 10.1128/jb.171.3.1278-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969 Nov;18(5):936–937. doi: 10.1128/am.18.5.936-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Ohman D. E. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J Bacteriol. 1988 Apr;170(4):1452–1460. doi: 10.1128/jb.170.4.1452-1460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Storey D. G., Hindahl M. S., Iglewski B. H. Differential regulation by iron of regA and toxA transcript accumulation in Pseudomonas aeruginosa. J Bacteriol. 1989 Oct;171(10):5304–5313. doi: 10.1128/jb.171.10.5304-5313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli D., Lottspeich F., Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990 Jun;4(6):895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Construction and characterization of Pseudomonas aeruginosa algB mutants: role of algB in high-level production of alginate. J Bacteriol. 1987 Apr;169(4):1593–1602. doi: 10.1128/jb.169.4.1593-1602.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhot C., Gicquel B., Davies J., Martín C. Isolation and analysis of IS6120, a new insertion sequence from Mycobacterium smegmatis. Mol Microbiol. 1992 Jan;6(1):107–113. doi: 10.1111/j.1365-2958.1992.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Chiodo V. A. Genetic and DNA sequence analysis of the Salmonella typhimurium virulence plasmid gene encoding the 28,000-molecular-weight protein. Infect Immun. 1990 Aug;58(8):2651–2658. doi: 10.1128/iai.58.8.2651-2658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. M., Brookes D. E., Stokes H. W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991 Aug;5(8):1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Hoiby N. Prevalence of mucoid strains of Pseudomonas aeruginosa in bacteriological specimens from patients with cystic fibrosis and patients with other diseases. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):549–552. [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J Bacteriol. 1992 Jun;174(11):3514–3521. doi: 10.1128/jb.174.11.3514-3521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josson K., Soetaert P., Michiels F., Joos H., Mahillon J. Lactobacillus hilgardii plasmid pLAB1000 consists of two functional cassettes commonly found in other gram-positive organisms. J Bacteriol. 1990 Jun;172(6):3089–3099. doi: 10.1128/jb.172.6.3089-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Misra T. K., Chakrabarty A. M. AlgR3, a protein resembling eukaryotic histone H1, regulates alginate synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2887–2891. doi: 10.1073/pnas.87.8.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaskar A. S., Reddy B. V. A method to locate protein coding sequences in DNA of prokaryotic systems. Nucleic Acids Res. 1985 Jan 11;13(1):185–194. doi: 10.1093/nar/13.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konyecsni W. M., Deretic V. DNA sequence and expression analysis of algP and algQ, components of the multigene system transcriptionally regulating mucoidy in Pseudomonas aeruginosa: algP contains multiple direct repeats. J Bacteriol. 1990 May;172(5):2511–2520. doi: 10.1128/jb.172.5.2511-2520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Aeration selects for mucoid phenotype of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):986–990. doi: 10.1128/jcm.24.6.986-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskiw B. K., Mevarech M., Barritt L. S., Jensen S. E., Henderson D. J., Hopwood D. A., Bruton C. J., Chater K. F. Discovery of an insertion sequence, IS116, from Streptomyces clavuligerus and its relatedness to other transposable elements from actinomycetes. J Gen Microbiol. 1990 Jul;136(7):1251–1258. doi: 10.1099/00221287-136-7-1251. [DOI] [PubMed] [Google Scholar]
- Lory S., Strom M. S., Johnson K. Expression and secretion of the cloned Pseudomonas aeruginosa exotoxin A by Escherichia coli. J Bacteriol. 1988 Feb;170(2):714–719. doi: 10.1128/jb.170.2.714-719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. W., Holloway B. W., Deretic V. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J Bacteriol. 1993 Feb;175(4):1153–1164. doi: 10.1128/jb.175.4.1153-1164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Kawahara K., Terakado N., Danbara H. Nucleotide sequence of a gene encoding a 29 kDa polypeptide in mba region of the virulence plasmid, pKDSC50, of Salmonella choleraesuis. Nucleic Acids Res. 1990 Feb 25;18(4):1055–1055. doi: 10.1093/nar/18.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T. B., Shinabarger D., Maharaj R., Kato J., Chu L., DeVault J. D., Roychoudhury S., Zielinski N. A., Berry A., Rothmel R. K. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991 Apr;4(2):191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Vasil M. L. Possible insertion sequences in a mosaic genome organization upstream of the exotoxin A gene in Pseudomonas aeruginosa. J Bacteriol. 1990 Apr;172(4):2020–2028. doi: 10.1128/jb.172.4.2020-2028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantschuk M., Richter G. Y., Mukhopadhyay P., Mills D. IS801, an insertion sequence element isolated from Pseudomonas syringae pathovar phaseolicola. Mol Microbiol. 1991 Mar;5(3):617–622. doi: 10.1111/j.1365-2958.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Stokes H. W., Hall R. M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989 Dec;3(12):1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun. 1991 Feb;59(2):471–477. doi: 10.1128/iai.59.2.471-477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Role of energy metabolism in conversion of nonmucoid Pseudomonas aeruginosa to the mucoid phenotype. Infect Immun. 1992 Apr;60(4):1329–1335. doi: 10.1128/iai.60.4.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Chamberlain C., Grant C. C. Molecular studies of Pseudomonas exotoxin A gene. Infect Immun. 1986 May;52(2):538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlleben W., Arnold W., Bissonnette L., Pelletier A., Tanguay A., Roy P. H., Gamboa G. C., Barry G. F., Aubert E., Davies J. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989 Jun;217(2-3):202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A., Bryan L. E., Storey D. G., Mattingly S. J., Vogel H. J., Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991 Jan;163(1):143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- Wozniak D. J., Ohman D. E. Pseudomonas aeruginosa AlgB, a two-component response regulator of the NtrC family, is required for algD transcription. J Bacteriol. 1991 Feb;173(4):1406–1413. doi: 10.1128/jb.173.4.1406-1413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zolg J. W., Hänggi U. J. Characterization of a R plasmid-associated, trimethoprim-resistant dihydrofolate reductase and determination of the nucleotide sequence of the reductase gene. Nucleic Acids Res. 1981 Feb 11;9(3):697–710. doi: 10.1093/nar/9.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D., Bron S., Venema G., Oskam L. Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res. 1989 Sep 25;17(18):7283–7294. doi: 10.1093/nar/17.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]