Abstract
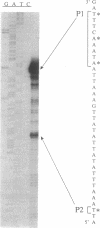
The pag gene of Bacillus anthracis, located on plasmid pXO1 (185 kb), encodes protective antigen, a component of the anthrax lethal and edema toxins. Synthesis of protective antigen is enhanced during growth of the organism with elevated levels of CO2. The CO2 effect is at the level of transcription, and pXO1-encoded regulatory factors have been implicated in control of pag expression. We used a Tn917-LTV3 insertion mutant of B. anthracis in which the wild-type pag gene on pXO1 was replaced with a pag-lacZ transcriptional fusion to monitor pag promoter activity. Expression of the pag-lacZ fusion is induced five- to eightfold during growth in 5% CO2 compared with growth in air. Growth in 20% CO2 increases transcription up to 19-fold. By monitoring pag-lacZ expression in atmospheres with different O2 and CO2 concentrations, we demonstrated definitively that the CO2 effect is specific and not simply a result of increased anaerobiosis. The results of 5' end mapping of pag transcripts indicate multiple sites of transcript initiation. We have determined two major apparent start sites, designated P1 and P2, located at positions -58 and -26 relative to the translation initiation codon, respectively. Analysis of total RNA from late-log-phase cells shows comparable initiation from P1 and P2 in wild-type strains grown in aerobic conditions. However, initiation from P1 is increased approximately 10-fold in cultures grown with an elevated level (5%) of CO2. We have identified a locus on pXO1, more than 13 kb upstream from the pag gene, which enhances pag transcription. When added in trans, this locus increases the level of transcripts with 5' ends mapping to P1 but has no effect on the level of transcripts with 5' ends mapping to P2. The CO2 effect on P1 is observed only in the presence of the activator locus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartkus J. M., Leppla S. H. Transcriptional regulation of the protective antigen gene of Bacillus anthracis. Infect Immun. 1989 Aug;57(8):2295–2300. doi: 10.1128/iai.57.8.2295-2300.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti L., Green B. D., Thorne C. B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985 May;162(2):543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg T. S., Robertson D. L. Nucleotide sequence and analysis of the lethal factor gene (lef) from Bacillus anthracis. Gene. 1989 Sep 1;81(1):45–54. doi: 10.1016/0378-1119(89)90335-1. [DOI] [PubMed] [Google Scholar]
- Camilli A., Portnoy A., Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990 Jul;172(7):3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Geist R. T., Perez-Casal J., Scott J. R. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J Bacteriol. 1992 Sep;174(17):5693–5701. doi: 10.1128/jb.174.17.5693-5701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi A., Fouet A., Mock M. Regulation of pag gene expression in Bacillus anthracis: use of a pag-lacZ transcriptional fusion. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):89–93. doi: 10.1016/0378-1097(92)90137-d. [DOI] [PubMed] [Google Scholar]
- Cataldi A., Labruyère E., Mock M. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol Microbiol. 1990 Jul;4(7):1111–1117. doi: 10.1111/j.1365-2958.1990.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuyer V., Duflot E., Sezer O., Danchin A., Mock M. Structural homology between virulence-associated bacterial adenylate cyclases. Gene. 1988 Nov 30;71(2):293–298. doi: 10.1016/0378-1119(88)90045-5. [DOI] [PubMed] [Google Scholar]
- Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. Demonstration of a capsule plasmid in Bacillus anthracis. Infect Immun. 1985 Aug;49(2):291–297. doi: 10.1128/iai.49.2.291-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Welkos S. L. Cloning and expression of the Bacillus anthracis protective antigen gene in Bacillus subtilis. Infect Immun. 1986 Nov;54(2):537–542. doi: 10.1128/iai.54.2.537-542.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Yamamoto K. New medium for the production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J Clin Microbiol. 1985 Sep;22(3):405–408. doi: 10.1128/jcm.22.3.405-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass E. H., Kendrick M. I., Tsai Y. C., Parsonnet J. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J Infect Dis. 1987 Apr;155(4):812–815. doi: 10.1093/infdis/155.4.812. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- MEYNELL E., MEYNELL G. G. THE ROLES OF SERUM AND CARBON DIOXIDE IN CAPSULE FORMATION BY BACILLUS ANTHRACIS. J Gen Microbiol. 1964 Jan;34:153–164. doi: 10.1099/00221287-34-1-153. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Uchida I., Terakado N., Yoshikawa M. Cloning and CO2-dependent expression of the genetic region for encapsulation from Bacillus anthracis. Mol Microbiol. 1988 May;2(3):371–376. doi: 10.1111/j.1365-2958.1988.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Makino S., Uchida I., Terakado N., Sasakawa C., Yoshikawa M. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol. 1989 Feb;171(2):722–730. doi: 10.1128/jb.171.2.722-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock M., Labruyère E., Glaser P., Danchin A., Ullmann A. Cloning and expression of the calmodulin-sensitive Bacillus anthracis adenylate cyclase in Escherichia coli. Gene. 1988 Apr 29;64(2):277–284. doi: 10.1016/0378-1119(88)90342-3. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Okada N., Geist R. T., Caparon M. G. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol. 1993 Mar;7(6):893–903. doi: 10.1111/j.1365-2958.1993.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Perez-Casal J., Caparon M. G., Scott J. R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991 Apr;173(8):2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Leppla S. H. Molecular cloning and expression in Escherichia coli of the lethal factor gene of Bacillus anthracis. Gene. 1986;44(1):71–78. doi: 10.1016/0378-1119(86)90044-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Tippetts M. T., Leppla S. H. Nucleotide sequence of the Bacillus anthracis edema factor gene (cya): a calmodulin-dependent adenylate cyclase. Gene. 1988 Dec 20;73(2):363–371. doi: 10.1016/0378-1119(88)90501-x. [DOI] [PubMed] [Google Scholar]
- Shimamura T., Watanabe S., Sasaki S. Enhancement of enterotoxin production by carbon dioxide in Vibrio cholerae. Infect Immun. 1985 Aug;49(2):455–456. doi: 10.1128/iai.49.2.455-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., BELTON F. C. An agar-diffusion method for titrating Bacillus anthracis immunizing antigen and its application to a study of antigen production. J Gen Microbiol. 1957 Oct;17(2):505–516. doi: 10.1099/00221287-17-2-505. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., GOMEZ C. G., HOUSEWRIGHT R. D. Synthesis of glutamic acid and glutamyl polypeptide by Bacillus anthracis. II. The effect of carbon dioxide on peptide production on solid media. J Bacteriol. 1952 Mar;63(3):363–368. doi: 10.1128/jb.63.3.363-368.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida I., Hornung J. M., Thorne C. B., Klimpel K. R., Leppla S. H. Cloning and characterization of a gene whose product is a trans-activator of anthrax toxin synthesis. J Bacteriol. 1993 Sep;175(17):5329–5338. doi: 10.1128/jb.175.17.5329-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida I., Makino S., Sasakawa C., Yoshikawa M., Sugimoto C., Terakado N. Identification of a novel gene, dep, associated with depolymerization of the capsular polymer in Bacillus anthracis. Mol Microbiol. 1993 Aug;9(3):487–496. doi: 10.1111/j.1365-2958.1993.tb01710.x. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Leppla S. H. Cloning of the protective antigen gene of Bacillus anthracis. Cell. 1983 Sep;34(2):693–697. doi: 10.1016/0092-8674(83)90402-6. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Lowe J. R., Eden-McCutchan F., Vodkin M., Leppla S. H., Schmidt J. J. Sequence and analysis of the DNA encoding protective antigen of Bacillus anthracis. Gene. 1988 Sep 30;69(2):287–300. doi: 10.1016/0378-1119(88)90439-8. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Crowther P. J., Doherty J., Jefferson S., DeCruz E., Noyer-Weidner M., Smith S. S., Michael M. Z., Graham M. W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989 May 11;17(9):3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]