Abstract
β-amyloid protein (Aβ) formation was reconstituted in permeabilized neuroblastoma cells expressing human Alzheimer β-amyloid precursor protein (βAPP) harboring the Swedish double mutation associated with familial early-onset Alzheimer disease. Permeabilized cells were prepared following metabolic labeling and incubation at 20°C, a temperature that allows βAPP to accumulate in the trans-Golgi network (TGN) without concomitant Aβ formation. Subsequent incubation at 37°C led to the generation of Aβ. Aβ production in the TGN persisted even under conditions in which formation of nascent post-TGN vesicles was inhibited by addition of guanosine 5′-O-(3-thiotriphosphate), a nonhydrolyzable GTP analogue, or by omission of cytosol. These and other results indicate that vesicle budding and trafficking may not be required for proteolytic metabolism of βAPP to Aβ, a process that includes “γ-secretase” cleavage within the βAPP transmembrane domain.
The β-amyloid protein (Aβ), a major component of parenchymal deposits in Alzheimer disease, is generated by proteolytic cleavage of the β-amyloid precursor protein (βAPP). Metabolism of the transmembrane βAPP occurs via alternative cellular trafficking and processing pathways (1). Cleavage of βAPP by “α-secretase” during, or after, transport to the cell surface generates a large amino-terminal fragment (sβAPPα) and precludes formation of Aβ (2). Cleavage of βAPP by “β-secretase” at the amino terminus of the Aβ domain yields sβAPPβ (3) and a potentially amyloidogenic carboxyl-terminal fragment. Presumably, this carboxyl-terminal fragment is subsequently cleaved within its transmembrane region by “γ-secretase” to generate the Aβ peptide (4–7). While intramembranous γ-secretase-type protein processing is highly unusual, a similar cleavage event has recently been shown to occur during maturation of sterol-regulatory element-binding protein-2 (8). In this study, a cell-free reconstitution assay was used to examine the vesicular compartment(s) involved in Aβ generation in mouse neuroblastoma cells (N2a) expressing the human “Swedish βAPP” variant (7). Unexpectedly, we observed conversion of βAPP to Aβ in this system even in the presence of inhibitors that appear to abolish vesicle budding. These data suggest that conventional dynamic vesicular events [e.g., processing during trafficking through a multivesicular body (9, 10)] may not be required for the γ-secretase-type intramembranous protein cleavage studied here. These data support the sterol-regulatory element-binding protein-2 results (8), which indicate that some proteinases may exert their actions on substrate sites localized within membranes.
MATERIALS AND METHODS
Intact Cell Studies.
N2a cells (3 confluent 35-mm dishes) expressing Swedish βAPP (7) were pulse-labeled with 500 μCi/ml [35S]methionine (NEN/DuPont) (1 Ci = 37 GBq) for 10 min at 37°C, preincubated for 2 hr at 20°C, and then incubated for 60 min at 20°C or 37°C. Cell lysates and culture media were precleared with βAPP carboxyl-terminal antibody 369 (11), and Aβ was detected by immunoprecipitation (12) with antibody 4G8 (13), followed by 10–20% tricine SDS/PAGE and autoradiography on Kodak X-OMAT AR5 film.
Preparation of Permeabilized N2a Cells.
Following a 10-min pulse-labeling at 37°C and a 2 hr incubation at 20°C (see above), cells (3 reaction aliquots per confluent 100-mm dish) were permeabilized as described (12). Briefly, labeled cells were treated at 4°C with hypotonic buffer (10 mM KCl/10 mM Hepes, pH 7.2) for 8 min and then broken by scraping with a rubber policeman in breaking buffer (90 mM KCl/20 mM Hepes, pH 7.2). This procedure resulted in >90% cell breakage, as evaluated by trypan blue staining. Permeabilized cells were then washed twice with 400 mM KOAc plus 20 mM Hepes and resuspended in 5 vol of breaking buffer.
Aβ Generation and Formation of Nascent Post-Trans-Golgi Network (TGN) Vesicles in Permeabilized N2a Cells.
Incubations were carried out for 90 min in a final volume of 300 μl, containing 120 μl permeabilized cells, 100 μl cytosol (12) (≈150 μg protein/ml), 2.5 mM MgCl2, 0.5 mM CaCl2, 110 mM KCl, an energy regenerating system (2 mM ATP/20 μM GTP/600 μM creatine phosphate/8 mg/ml creatine phosphate kinase), and a mixture of protease inhibitors (12). These incubations were carried out either (i) at 37°C under standard conditions, (ii) at 20°C under standard conditions, (iii) at 37°C in the absence of added cytosol, or (iv) at 37°C in the presence of 30 μM guanosine 5′-O-(3-thiotriphosphate) (GTPγS) (Boehringer Mannheim). For GTPγS experiments, permeabilized cells were preincubated with GTPγS at 4°C for 10 min prior to addition of the energy regenerating system.
Following incubation, permeabilized cells were centrifuged at 14,000 × g for 60 sec at 4°C. This procedure was previously demonstrated to separate the TGN from nascent post-TGN vesicles, which fractionate into pellet and supernatant, respectively (12, 14). Samples of pellets and supernatants were then analyzed for Aβ as described above. In some experiments, antibody 6E10 (13) was shown to immunoprecipitate from pellet fractions a radioactive band having the same migration rate as that immunoprecipitated by antibody 4G8, consistent with our designation of this band as AB. In control experiments, treatment of permeabilized cells with proteinase K (25 μg/ml) (Boehringer Mannheim) after the 90 min incubation resulted in digestion of Aβ-immunoreactive material in the presence, but not in the absence, of 1% Triton X-100, consistent with the predicted existence of Aβ within a vesicular lumen. In some experiments, the post-TGN vesicle fraction (supernatant) was further incubated for up to 90 min prior to analysis for Aβ.
In a separate series of experiments, designed to test the ability of GTPγS to prevent budding of vesicles from the TGN, cells were preincubated with [35S]sulfate for 5 min at 37°C, a procedure that labels proteins exclusively in the TGN. Permeabilized cells were then incubated in the absence or presence of GTPγS and fractionated as described above.
Equilibrium Density Sucrose Gradients.
Cells (one confluent 100-mm dish per reaction) were permeabilized as described. Incubations were carried out at 20°C under standard conditions, or at 37°C either under standard conditions or in the presence of 30 μM GTPγS or 1 μM bafilomycin A1 (baf A1) (Kamiya Biomedical, Thousand Oaks, CA). Cells were then homogenized using a stainless steel ball bearing homogenizer (19 μm clearance) (12), and TGN-enriched fractions were isolated using a step-wise sucrose gradient (12, 15). Each fraction was then analyzed for Aβ as described.
Densitometry.
Autoradiographic densities were quantitated using a Bio-Rad PhosphorImager (Molecular Dynamics) software version 2.0.
RESULTS
Conversion of Swedish βAPP to Intracellular Aβ in Intact and Permeabilized Cells.
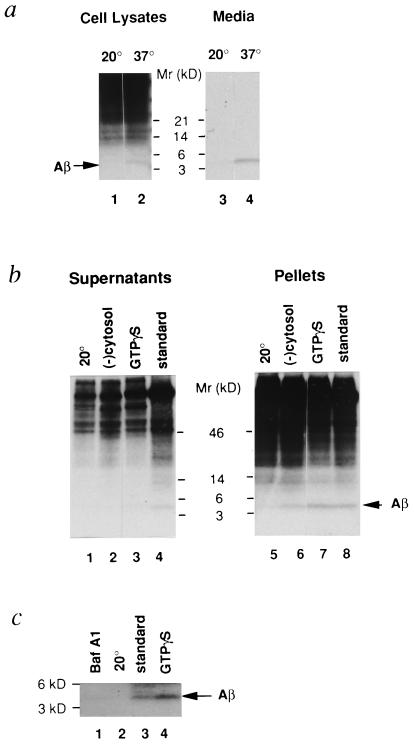
Intact cells were pulse-labeled at 37°C for 10 min and chased at either 20°C or 37°C for 2 hr. It is well established that a 20°C block results in accumulation of secretory and membrane proteins in the TGN (12, 16, 17) and inhibition of prohormone processing (12). As expected, when cells were incubated at 20°C, we failed to detect Aβ in cell lysates or media (Fig. 1a, lanes 1 and 3). These findings are consistent with earlier studies (17) and indicate that βAPP accumulated in the TGN fails to be metabolized to Aβ at 20°C. On the other hand, Aβ was clearly evident in lysates and media of cells incubated at 37°C (Fig. 1a, lanes 2 and 4).
Figure 1.
Aβ formation from Swedish βAPP in intact cells (a), permeabilized cells (b), and purified TGN (c). (a) A 20°C block prevents Aβ generation in intact cells. N2a cells expressing Swedish βAPP were pulse-labeled with [35S]methionine at 37°C for 10 min and then further incubated at either 20°C (lanes 1 and 3) or 37°C (lanes 2 and 4) for 2 hr. Samples of medium and cell lysate were immunoprecipitated with antibody 4G8 and analyzed on 10–20% Tricine SDS/PAGE. Arrow indicates Aβ peptide. (b) Aβ generation in permeabilized cells. Cells were pulse-labeled with [35S]methionine at 37°C for 10 min and incubated at 20°C for 2 hr. Permeabilized cells were then prepared as described. Aliquots of permeabilized cells were incubated for 90 min either under standard conditions at 20°C (lanes 1 and 5) or 37°C (lanes 4 and 8), or at 37°C in the absence of added cytosol (lanes 2 and 6), or at 37°C in the presence of 30 μM GTPγS (lanes 3 and 7). Following fractionation, samples of supernatant and pellet were immunoprecipitated with antibody 4G8 and analyzed on 10–20% Tricine SDS/PAGE. (c) Aβ generated in permeabilized cells is recovered in a TGN-enriched fraction. Permeabilized cells that were previously incubated for 90 min at 20°C under standard conditions (lane 2) or at 37°C either under the standard conditions (lane 3) or in the presence of 1 μM baf A1 (lane 1) or 30 μM GTPγS (lane 4) were homogenized, and the postnuclear supernatant was applied to an equilibrium sucrose gradient (12). The TGN-enriched fractions were immunoprecipitated with antibody 4G8 and analyzed on 10–20% Tricine SDS/PAGE.
We then tested whether Aβ could be generated in a well-established cell-free system (12). To this end, N2a cells expressing Swedish βAPP were pulse-labeled at 37°C for 10 min, followed by a 20°C incubation for 2 hr to allow full-length βAPP to accumulate in the TGN. Permeabilized cells were then prepared and incubated under various conditions as described in Materials and Methods. As in intact cells, Aβ was generated upon incubation of permeabilized cells under standard conditions at 37°C, whereas very little was generated at 20°C (Fig. 1b). Incubation at 37°C resulted in the appearance of Aβ in both the fraction containing the Golgi/TGN (pellet) and the fraction containing nascent post-TGN vesicles (supernatant) (Fig. 1b). Immunoprecipitation using antibody 4G8, followed by mass spectrometry analysis (18), revealed that Aβ present in both fractions was primarily Aβ1–40 (data not shown).
Subcellular Localization of β- and γ-Secretase Cleavages of Swedish βAPP.
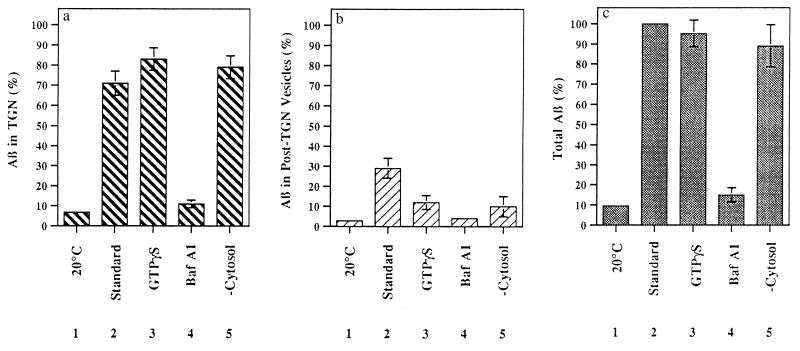
To distinguish between the Golgi/TGN and post-TGN vesicles as the site of formation of Aβ, we performed cell-free reactions under conditions designed to prevent formation of nascent post-TGN vesicles. The level of Aβ in nascent post-TGN vesicles was greatly reduced when cytosol was omitted from the reaction mixture, whereas Aβ in the TGN-containing fraction was unaffected (Figs. 1b and 2). The addition of GTPγS, a nonhydrolyzable GTP analogue, to the permeabilized cell preparation diminished the level of Aβ in post-TGN vesicles. Aβ levels in the TGN-containing fraction were slightly increased (Figs. 1b and 2; see also Fig. 1c). In view of earlier studies indicating that budding of vesicles from the TGN requires the presence of factors in the cytosol (19) and is inhibited by GTPγS (12, 20), our data indicate that Aβ can be formed from Swedish βAPP prior to vesicle budding from the TGN. In support of this view, addition of GTPγS to permeabilized cells abolished the appearance of all [35S]-sulfated proteins in the post-TGN vesicle fraction (data not shown).
Figure 2.
Quantitative analysis of Aβ formation in permeabilized cells. Cells were incubated for 90 min at 20°C under standard conditions or at 37°C under the conditions indicated. The histograms show the amount of Aβ present in the TGN (a), in post-TGN nascent vesicles (b), and in TGN plus post-TGN nascent vesicles (c). Autoradiographic densities were quantitated using a Bio-Rad PhosphorImager, software version 2.0. In each experiment, data were normalized to total Aβ formed under standard conditions. Data represent means ± SEM for three experiments.
To evaluate further the possibility that Aβ was produced in the TGN, we prepared a TGN-enriched fraction from incubated permeabilized cells using a well-established sucrose gradient protocol (12, 15). Aβ, generated in permeabilized cell preparations under standard conditions at 37°C, was recovered in this TGN-enriched fraction (Fig. 1c, lane 3); even higher levels of Aβ were recovered when cells were incubated in the presence of GTPγS, presumably due to reduced Aβ export from the TGN (Fig. 1c, lane 4). Little or no Aβ was detectable in TGN-enriched fractions prepared from permeabilized cells incubated at 20°C under standard conditions (Fig. 1c, lane 2). Earlier studies in intact cells documented that soluble Aβ production from the Swedish βAPP variant is inhibited by baf A1, a proton ATPase inhibitor (21), indicating that Aβ is generated in an acidic compartment (22, 23). Aβ production in the permeabilized cell system (Fig. 2) and in the TGN-enriched fraction prepared from this system (Fig. 1c) was also greatly reduced by baf A1. Because the TGN, a well-defined acidic compartment in the secretory pathway (24, 25), is the major acidic organelle in the TGN-enriched fraction, it is the primary target for baf A1 in this fraction.
In another series of experiments, we tested the ability of the post-TGN vesicle fraction, obtained following 90 min of incubation of permeabilized cells, to produce Aβ. Incubation of this fraction for periods of 30–90 min failed to generate additional Aβ, further suggesting the Golgi/TGN rather than the post-TGN vesicles as the site of Aβ formation (data not shown).
Time- and Temperature-Dependence of Aβ Generation in Permeabilized Cells.
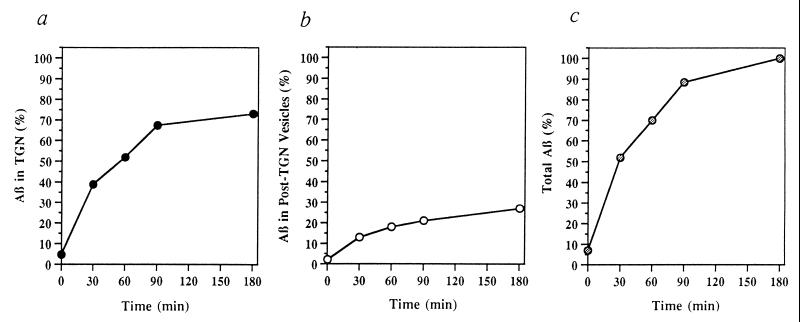
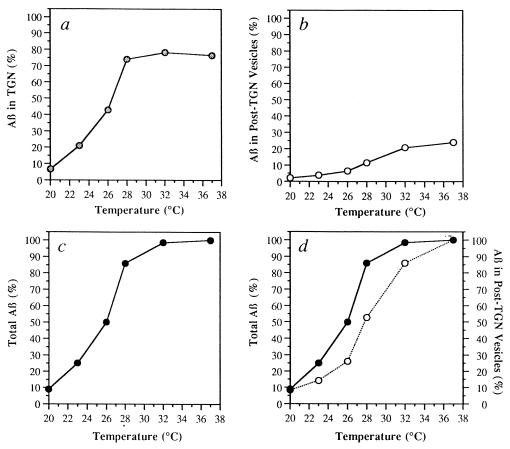
Time- and temperature-dependence of Aβ formation in permeabilized cells were also examined. Permeabilized cell preparations were incubated under standard conditions either at 37°C for various times (Fig. 3) or at different temperatures ranging from 20°C to 37°C for 90 min (Fig. 4). Both formation of Aβ and its appearance in the vesicle fraction were near-maximal at ≈90 min (Fig. 3) and near-optimal at 32°C–37°C (Fig. 4). Moreover, the diminished rate of budding of Aβ transport vesicles relative to formation of Aβ (calculated as the sum of Aβ in the TGN plus Aβ in post-TGN vesicles) over the temperature range from 23°C to 32°C supports the view that cleavage of βAPP to form Aβ and budding of Aβ transport vesicles are distinct cellular events (Fig. 4d).
Figure 3.
Time dependence of Aβ formation in permeabilized cells. Aβ present in the TGN (a), post-TGN nascent vesicles (b), and TGN plus post-TGN nascent vesicles (c) is shown as a function of incubation time at 37°C. Data represent means for two experiments.
Figure 4.
Temperature dependence of Aβ formation in permeabilized cells. Aβ present in the TGN (a), post-TGN nascent vesicles (b and d) and TGN plus post-TGN nascent vesicles (c and d) is shown following incubation for 90 min at the indicated temperature. (a–c) In each experiment, data were normalized to total Aβ formed at 37°C. (d) In each experiment, data representing total Aβ (•) and Aβ in the post-TGN nascent vesicles (○) were normalized to their respective values at 37°C. Data represent means for two experiments.
DISCUSSION
Previous data indicated that Swedish βAPP is cleaved by β-secretase in the Golgi apparatus (7). This conclusion was confirmed and extended by the present evidence, suggesting that Swedish βAPP is cleaved by both β-secretase and γ-secretase activities within the Golgi apparatus, and more specifically, within the TGN. The demonstration that Aβ is formed intracellularly in differentiated NT2N cells (26) suggests physiological relevance for the our studies in which undifferentiated N2a cells were used. Interestingly, the formation of Aβ within the TGN was exquisitely sensitive to temperature. Hence, we hypothesize that the inhibition of β-secretase cleavage of Swedish βAPP observed at 20°C in an earlier report (17) reflects a reduction in β-secretase activity of the Golgi/TGN at that temperature.
Several lines of evidence indicate that β-secretase activities act at multiple subcellular sites and may have distinct preferences for cleavage of wild-type βAPP vs. Swedish βAPP (7, 27–29). We and others have characterized Aβ production from the Swedish βAPP variant because of the technical limitations inherent in measuring the exceedingly small amounts of Aβ generated from wild-type βAPP. When sufficiently sensitive methods are developed, it will be important to establish the extent to which β-secretase and/or γ-secretase enzymes in the Golgi/TGN are also responsible for the formation of Aβ from wild-type βAPP.
The most surprising result that emerges from our studies is that γ-secretase cleavage occurs even in the absence of detectable budding (e.g., when vesicle budding is inhibited by the addition of GTPγS or by the omission of cytosol). We previously postulated that γ-secretase cleavage might occur during trafficking of βAPP through the internal vesicles of a multivesicular body (10), an organelle encountered by a variety of membrane proteins (9). Once inside the multivesicular body, breach of the bilayer of βAPP-containing internal vesicles would provide access of lumenal proteinases to the intramembranous domain of βAPP where γ-secretase-like cleavage could yield the C termini of Aβ1–40 and Aβ1–42(43).
These data support the suggestion (8) of a physiological mechanism for the cleavage of membrane proteins within their intramembranous domains. Given the complex nature and biophysical constraints of enzyme–substrate interactions involving the existence of proteinases which process substrates within lipid bilayers, efforts are now underway to reconstitute βAPP metabolism and Aβ generation in a genetically tractable system, Saccharomyces cerevisiae. Current evidence indicates that α-secretase-type βAPP metabolism can indeed be reconstituted in S. cerevisiae (30), including responsiveness of α-secretase metabolism to phorbol esters (M. Seeger, H. Komano, R. Fuller, P.G., and S.G., unpublished observations). Preliminary data demonstrating the generation of Aβ by Pichia pastoris (31) support the feasibility of this approach in lower organisms.
In conclusion, our data indicate the formation of Aβ in the TGN in a cell-free system. The use of cell-free systems (see also ref. 32) should accelerate progress toward the elucidation of the molecular machinery responsible for Aβ formation and toward the development of therapeutic agents that arrest or prevent the accumulation of Aβ.
Acknowledgments
This work was supported by U.S. Public Health Service Grants AG11508 (to S.G.) and AG09464 (to P.G.), by the C. V. Starr Foundation (to S.G.), by a grant from the American Foundation for Aging Research (to H.X.), by an Alzheimer’s Association/Mrs. Florence Martin Pilot Research Grant (to R.W.), by grants from the Adler Foundation (to S.S.S.), and by a Zenith Award from the Alzheimer’s Association (to S.S.S.).
ABBREVIATIONS
- Aβ
β-amyloid protein
- βAPP
Alzheimer β-amyloid precursor protein
- TGN
trans-Golgi network
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- baf A1
bafilomycin A1
References
- 1.Gandy S, Greengard P. Int Rev Neurobiol. 1994;36:29–50. doi: 10.1016/s0074-7742(08)60302-5. [DOI] [PubMed] [Google Scholar]
- 2.Sisodia S S, Koo E H, Beyreuther K, Unterbeck A, Price D L. Science. 1990;248:492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- 3.Seubert P, Oltersdorf T, Lee M G, Barbour R, Blomquist C, Davis D L, Bryant K, Galasko D, Thal L J, Fritz L, Lieberburg I, Schenk D. Nature (London) 1993;361:260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- 4.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D J, Lieberburg I, Schenk D. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 5.Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X, McKay D M, Tintner R, Frangione B, Younkin S G. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 6.Haass C, Schlossmacher M G, Hung A Y, Vigo-Pelfrey C, Mellon A, Ostaszewski B C, Lieberburg I, Koo E H, Schenk D, Teplow D, Selkoe D J. Nature (London) 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 7.Thinakaran G, Teplow D S, Siman R, Greenberg B, Sisodia S S. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 8.Sakai J, Duncan E A, Rawson R B, Hua X, Brown M S, Goldstein J L. Cell. 1996;85:1037–1046. doi: 10.1016/s0092-8674(00)81304-5. [DOI] [PubMed] [Google Scholar]
- 9.Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins C R. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- 10.Gandy S E, Buxbaum J D, Greengard P. In: Alzheimer’s Disease: New Treatment Strategies. Khachaturian Z S, Blass J P, editors. New York: Dekker; 1991. pp. 175–192. [Google Scholar]
- 11.Buxbaum J D, Gandy S E, Cicchetti P, Ehrlich M E, Czernik A J, Fracasso R P, Ramabhadran T V, Unterbeck A J, Greengard P. Proc Natl Acad Sci USA. 1990;87:6003–6007. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Shields D. J Cell Biol. 1993;122:1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K S, Wen G Y, Bancher C, Chen C J M, Sapienza V J, Hong H, Wisniewski H M. Neurosci Res Commun. 1990;7:113–122. [Google Scholar]
- 14.Musch A, Xu H, Shields D, Rodriguez-Boulan E. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell K E, Palade G E. J Cell Biol. 1982;92:822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matlin K S, Simons K. Cell. 1983;34:233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- 17.Haass C, Lemere C A, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe D J. Nat Med. 1995;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Sweeney D, Gandy S E, Sisodia S S. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 19.Rothman J E. Nature (London) 1994;372:55–62. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 20.Tooze S A, Weiss U, Huttner W B. Nature (London) 1990;347:207–208. doi: 10.1038/347207a0. [DOI] [PubMed] [Google Scholar]
- 21.Bowman E J, Siebers A, Altendorf K. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haass C, Capell A, Citron M, Teplow D B, Selkoe D J. J Biol Chem. 1995;270:6186–6192. doi: 10.1074/jbc.270.11.6186. [DOI] [PubMed] [Google Scholar]
- 23.Knops J, Suomensaari S, Lee M, McConlogue L, Seubert P, Sinha S. J Biol Chem. 1995;270:2419–2422. doi: 10.1074/jbc.270.6.2419. [DOI] [PubMed] [Google Scholar]
- 24.Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell S K, Quinn D L, Moore H P. Cell. 1987;51:1035–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Shields D. J Biol Chem. 1994;269:22875–22881. [PubMed] [Google Scholar]
- 26.Wertkin A M, Turner R S, Pleasure S J, Golde T E, Younkin S G, Trojanowski J Q, Lee V M-Y. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Citron M, Teplow D, Selkoe D J. Neuron. 1995;14:661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 28.Martin B L, Schrader-Fischer G, Busciglio J, Duke M, Paganetti P, Yankner B A. J Biol Chem. 1995;270:26727–26730. doi: 10.1074/jbc.270.45.26727. [DOI] [PubMed] [Google Scholar]
- 29.Perez R G, Squazzo S L, Koo E H. J Biol Chem. 1996;271:9100–9107. doi: 10.1074/jbc.271.15.9100. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Komano H, Fuller R, Gandy S, Frail D. J Biol Chem. 1994;269:27799–27802. [PubMed] [Google Scholar]
- 31.Le Brocque D S, Evin G, Henry A, Cappai R, Fuller S, Beyreuther K, Masters C L. Neurobiol Aging. 1996;17:S98–S99. [Google Scholar]
- 32.Desdouits F, Buxbaum J D, Desdouits-Magnen J, Nairn A C, Greengard P. J Biol Chem. 1996;271:24670–24674. doi: 10.1074/jbc.271.40.24670. [DOI] [PubMed] [Google Scholar]