Abstract
In confluent fibroblast monolayers, an increase in the selective uptake of free cholesterol (FC) from plasma low density lipoprotein (LDL) was accompanied by an increase in FC efflux. The rate of FC efflux was proportional to the FC content of the cell surface caveolae and to mRNA levels of caveolin, an FC-binding protein of caveolae. Inhibitors of LDL–FC internalization reduced the increase in caveolin mRNA levels and FC efflux. Oxysterols reduced caveolin mRNA levels, as well as transport of FC to the cell surface and FC efflux. DNA antisense to caveolin reduced caveolin mRNA and inhibited FC efflux. These data suggest that regulation of FC efflux can contribute to cellular FC homeostasis when LDL levels are modified over the physiological range, and they link this regulation to caveolin.
In peripheral cells, including fibroblasts, high-affinity low density lipoprotein (LDL) receptors are strongly down-regulated in media containing even low concentrations of plasma LDL (1). Few intact LDL particles are then taken up, but free cholesterol (FC) is selectively transferred from LDL into the cell (2). When extracellular LDL levels and the rate of selective LDL–FC influx were increased, cellular cholesterol mass changed only slightly, because FC efflux became correspondingly greater. The only significant initial acceptor of cell-derived FC in these experiments was plasma high density lipoprotein (HDL) (3).
Caveolae are clathrin-free cell surface invaginations, rich in FC and glycolipids. They are particularly abundant in adipocytes, vascular smooth muscle, endothelium, and fibroblasts (4). Several lines of evidence now identify caveolae as probable intermediates in the HDL-specific FC efflux pathway. Only FC within caveolae is a substrate for cholesterol oxidase in intact cells, suggesting that these organelles contain a major part of the FC directly accessible to the extracellular medium in the plasma membrane bilayer (5). When fibroblasts were labeled with 3H-FC from purified LDL, a large fraction of 3H-FC became available for oxidation, indicating preferential transfer of LDL-derived FC to the caveolae. The caveolar 3H-FC pool decreased rapidly when native plasma or HDL was present in the medium. LDL alone was an ineffective acceptor. In the presence of okadaic acid, an inhibitor of caveolar expression at the cell surface (6), FC efflux decreased (3).
Caveolin, a 22-kDa lipid-binding protein associated with caveolae, is a member of a gene family broadly implicated in transmembrane signaling and transport (4, 7). Its association with bilayer membranes requires the presence of FC (8, 9). Caveolin copurified with 3H-FC internalized from LDL (3). Some cells, including lymphocytes, hepatocytes, and many transformed cell lines, lack caveolin and caveolae (10–12). In these cells, FC and glycolipids are considered to form lipid “rafts” mobile within the plane of the plasma membrane (11). Transfection of lymphoid cells with caveolin cDNA confined these lipid rafts into typical caveolar pits (13). When FC was reduced in fibroblasts cultured in lipoprotein-deficient media, caveolin disappeared from the cell surface (14). These data suggested a functional relationship between caveolin, cellular FC transport, caveolae, and FC efflux.
Oxysterols have also been implicated in the regulation of FC efflux. These are formed from FC when it combines with oxygen, spontaneously or in reaction with tissue lipoxygenases. Cholesterol epoxides are the major oxysterols in minimally modified LDL (15). 7-Ketocholesterol (7-KC) and 7α-hydroxycholesterol (7αOH-C) are major components of the oxysterols in human atherosclerotic lesions (16). 7-KC was recently shown to reduce FC efflux from macrophages (17).
From the evidence linking caveolin and FC transport, it seemed possible that FC and oxysterols could modify FC efflux by means of opposite effects on the expression of caveolin. The influx and efflux of FC from LDL by the selective pathway is stimulated in confluent fibroblasts by increased medium LDL concentration (2). This made these cells a convenient model in which to study effects of caveolin on the regulation of FC efflux.
EXPERIMENTAL PROCEDURES
Materials.
FC and cholesterol α-epoxide (CαEP) were purchased from Sigma. 7αOH-C and cholesterol β-epoxide (CβEP) were from Research Plus (Bayonne, NJ). 7K-C was from Steraloids (Wilton, NH). [1,2-3H]Cholesterol (40–55 Ci·mmol−1; 1 Ci = 37 GBq) was from New England Nuclear. Sterols were >98% homogeneous, as determined by thin-layer chromatography on silica gel layers developed in petroleum ether/diethyl ether/acetic acid (80/20/1, vol/vol) or benzene/ethyl acetate (3/2, vol/vol). Cytochalasin D, included in some incubations, was from CalBiochem.
Cell Culture.
Normal skin fibroblasts were cultured to near confluence in 3.5-cm plastic dishes in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum. Cell FC mass, measured fluorimetrically (18) was 10–12 μg of FC per dish. Cholesteryl ester was undetectable under these conditions. Twenty-four hours before use, the cells were transferred into DMEM containing 7% (vol/vol) normolipemic human plasma prelabeled with [1,2-3H]cholesterol to a final specific activity of 2–6 × 104 cpm·μg−1 (19).
Purification of LDL and LDL-Free Plasma.
Blood was collected into ice-cooled tubes from normolipemic donors who had fasted overnight. These contained streptokinase (final concentration of 150 international units·ml−1) as anticoagulant. LDL was affinity-purified from plasma as previously described (20). All steps were carried out at 2–4°C. Briefly, native plasma was passed through columns of heparin-agarose (Sepharose 6B-heparin) (Sigma) equilibrated in Dulbecco’s phosphate-buffered saline, pH 7.4 (PBS) (20). In the nonadsorbed fraction, >98% of FC was recovered with HDL within density limits 1.063–1.21 g·ml−1. The adsorbed fraction containing LDL was eluted with 3 M NaCl/PBS (pH 7.4). In the adsorbed fraction, >95% of FC was recovered within density limits 1.019–1.063 g·ml−1. After dialysis overnight at 2–4°C and concentration into PBS containing 0.05 mM EDTA by centrifugal dialysis (Filtron, Northborough, MA), LDL or the nonadsorbed fraction containing HDL was used immediately in individual experiments.
In some experiments, unlabeled FC or oxysterols (2–4 mg·ml−1 in ethanol solution) were added over 5–10 min to native plasma at 37°C, to a final concentration of 10% (wt/wt) relative to plasma FC. Final ethanol concentration was <2%, conditions shown previously to be without effect on cellular FC transport (2). In other experiments, oxysterol was first complexed with albumin-agarose covalent complex (19) then incubated (60 min, 37°C) with unlabeled isolated LDL from heparin-agarose affinity chromatography. Oxysterol-modified LDL prepared by either method had the same biological properties in the assays described below. The distribution of oxysterol between plasma lipoproteins, and its recovery in LDL, were confirmed after thin-layer chromatography. In a representative experiment, recovery of total CαEP in LDL (70 ± 2%) did not differ from recovery of FC (72 ± 4%) in the same fraction after affinity purification on heparin-agarose.
Selective FC Transfer from LDL.
Native plasma was labeled with 3H-FC (19). Part was diluted to 7% (vol/vol) with DMEM to label fibroblast monolayers for 24 h at 37°C. The remainder was kept on ice. After 24 h, the cells were transferred to DMEM containing 80% (vol/vol) of the same labeled plasma preparation for 0–12 h. In some experiments, LDL from the same labeled plasma isolated by heparin affinity chromatography, or the nonadsorbed fraction containing HDL but not LDL, was used. This protocol ensured that the specific activities of FC in native plasma (7% or 80%) and cells were equivalent. This was confirmed from the FC specific activities of each, which were consistent ±5% in the same experiment. As a result, a change over time in the rate at which FC label appeared in the medium reflected a proportional change in the rate of FC efflux.
At intervals dishes were transferred into 80% plasma or its fractions, and cells were processed to determine caveolar FC, the level of caveolin mRNA, or FC efflux, using protocols described below.
Measurement of Caveolar FC.
Dishes of labeled cells were washed four times at 2–4°C with PBS. Each dish was incubated with unlabeled LDL in PBS to exchange with cell-surface 3H-labeled LDL particles (21). The cells were washed again with PBS containing human serum albumin (5 mg·ml−1; Sigma) and finally four times with PBS. To determine the mass of FC associated with the caveolae, cell monolayers were incubated (4 h, 0–2°C) with cholesterol oxidase (final concentration 1 unit·ml−1). These conditions selectively modify caveolar FC (21). The cells were dissolved in 0.1 M NaOH and were extracted with chloroform and methanol. Portions of chloroform phase were fractionated by thin-layer chromatography to separate labeled cholest-4-en-3-one produced by cholesterol oxidase from unmodified FC (3). FC mass accessible to cholesterol oxidase was calculated from cholest-4-en-3-one label, divided by cellular FC specific activity.
Measurement of FC Efflux.
Efflux was measured using cells washed as described above, as the rate at which cellular 3H-FC label was transferred to extracellular medium containing 80% unlabeled native plasma and 20% DMEM (37°C, 3 min). Over this period FC efflux was linear (22). Samples of medium were cooled in ice water, and immediately centrifuged (2000 × g, 10 min, 4°C). FC radioactivity was measured on portions of supernatant by liquid scintillation spectrometry. Efflux rates were calculated from medium label and cellular FC specific activity.
In some experiments the fibroblast monolayers had been preincubated with hyperosmotic medium in which the NaCl concentration in PBS was raised to 350 mM by addition of solid NaCl (23) or with cytochalasin D (40 μM) (21), 30 min prior to and during incubation with labeled 80% (vol/vol) native plasma or LDL.
Cloning and Sequencing of Caveolin cDNA.
A human lung cDNA library (gift of Carol Basbaum, University of California, San Francisco) was screened with a 25-mer synthetic oligonucleotide antisense to the 5′ and 3′ termini of the published base sequence of human caveolin (10). Four full-length clones were identified. Sequencing was carried out by the dideoxynucleotide method (24). Three differences from the expected base sequence were consistently found. In codon 16, GTT → GCT substituted Ala for Val. In codon 52, GTC → GTT was conservative. In codon 144, ACC → ATC substituted Ile for Thr. Both substitutions have been previously identified in the caveolin sequence of other species. These changes probably represent spontaneous mutations in the human caveolin sequence (25, 26).
Northern Blotting of Caveolin mRNA.
Total RNA was purified from fibroblast monolayers with RNeasy kits (Qiagen, Chatsworth, CA). Cells in one 6-cm dish or three 3.5-cm dishes yielded ≈20 μg of total RNA. For Northern blotting, either 4 or 6 μg of RNA was electrophoresed on a 1% agarose/formaldehyde denaturing gel. An equal mass of RNA was added to all lanes of a gel. After electrophoresis, RNA was transferred to a nylon membrane by capillary blotting. Each blot was hybridized to full-length random-primed 32P-labeled caveolin cDNA. Both prehybridization and hybridization were carried out at 65°C. Blots were visualized on Kodak X-Omat AR film and scanned with a computerized densitometer (ImageQuant; Molecular Dynamics), under conditions where the signal was linear with amount of sample applied. Annexin-6, a caveolar protein without known activity in FC transport (27), served as control, and was invariant within all experiments. A 30-mer oligonucleotide (5′-GCCAATGCCCGAGATGGCATCTTTAATTTC-3′) antisense to codons 100–109 of human annexin-6 (28) was labeled and used for Northern blots as described above.
Effects of Antisense Oligonucleotides.
A phosphorothioate-substituted 24-mer (5′-GTATTTGCCCCCAGACATGCTGGC-3′) antisense to caveolin cDNA nucleotides 22–45 (10) was synthesized by Operon Technologies (Alameda, CA). The equivalent sense strand served as positive control. Approximately 107 fibroblasts from nearly confluent monolayers were trypsinized, washed with DMEM, then suspended in 0.5 ml of medium (OptiMEM I; GIBCO/BRL) containing 50 or 100 μg of sense or antisense oligonucleotide. Control suspensions contained cells without oligonucleotide. Electroporation was carried out at 25°C using a Gene Pulser II (Bio-Rad) at 220 V and 960 μF. Treated cells were incubated at 37°C for 4 days. Monolayers were labeled to equilibration in medium containing 3H-FC plasma. Individual dishes were then used to determine either caveolin mRNA levels or FC efflux rates, using the protocols described above.
RESULTS
Effects of Plasma Concentration on FC Efflux.
Native plasma was labeled with 3H-FC as described in Experimental Methods. Fibroblast monolayers were preequilibrated in 7% (vol/vol) 3H-FC labeled native plasma/DMEM, thus ensuring that the specific activities of FC in whole plasma and cells were equal. At zero time, the cells were washed with PBS and transferred to medium containing 80% (vol/vol) of the same labeled plasma. Under these conditions the transfer of label from cells to medium reflects a proportional change in rate of FC efflux at steady state.
At each time point indicated in Fig. 1 Upper dishes of labeled cells were washed and transferred to unlabeled 80% (vol/vol) plasma medium for 3 min at 37°C, to determine the rate at which cellular FC label was transferred out of the cell. As previously described (3), under these conditions the FC efflux was linear. Because the specific activities of cells and medium were equivalent, the rate of appearance of 3H-FC in the medium, divided by FC specific activity, reflected the rate at which cellular FC was transferred to the medium.
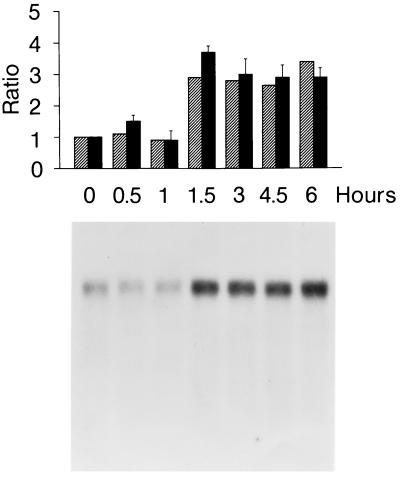
Figure 1.
Time course of the induction of caveolin mRNA and FC efflux. Cells equilibrated in 3H-FC-labeled 7% (vol/vol) plasma/DMEM were transferred at zero time into labeled 80% (vol/vol) plasma medium. At the intervals shown, monolayers were collected for extraction of RNA and estimation of caveolin mRNA levels. At the same intervals, cells were washed, then transferred into 80% (vol/vol) unlabeled plasma medium to measure FC efflux. (Upper) Hatched bars, caveolin mRNA levels, relative to zero time, determined by densitometry. Each lane contained 4 μg of total RNA. Solid bars, FC efflux rates, relative to zero time. The correlation coefficient between fold stimulation of FC efflux and mRNA was 0.91. (Lower) Northern blots of caveolin mRNA over the same time course.
At zero time, the rate of FC efflux into the unlabeled plasma was 28 ± 6 ng·min−1 (n = 6), similar to values previously reported (2). This rate increased ≈3.5-fold over 3 h after transfer of the cells from 7% (vol/vol) to 80% (vol/vol) 3H-FC labeled plasma. The increase at 4.5 h and 6 h was (2.9 ± 0.5)- and (2.9 ± 0.3)-fold, respectively
Effects of Plasma Concentration on Caveolin mRNA Levels.
Using the same protocol as in the preceding section, we determined caveolin mRNA levels as a function of time after the cells were transferred from 7% (vol/vol) to 80% (vol/vol) plasma/DMEM. As shown in Fig. 1 Lower, changes in caveolin mRNA levels were similar in extent and followed a time course parallel to the time course in FC efflux.
When affinity-purified LDL was added at 80% (vol/vol) relative to its concentration in plasma, in place of native plasma, caveolin mRNA levels were increased [(3.0 ± 0.6)-fold, n = 4]. On the contrary, when the cells were transferred into 80% (vol/vol) plasma from which LDL had been removed with heparin-agarose, there was no detectable rise in caveolin mRNA levels These results suggested that LDL was the major plasma factor responsible for the up-regulation of caveolin mRNA. Annexin-6 mRNA levels were not modified. For example, at 3 h after transfer from 7% to 80% plasma medium, annexin-6 mRNA levels were (1.1 ± 0.1)-fold those measured at zero time.
Inhibition of FC Efflux and Caveolin mRNA.
Inhibitors previously shown to block the uptake of FC from LDL (2, 21) were tested for their effects on the stimulation of caveolin mRNA levels and on FC efflux. Both were measured 3 h after transfer of the cells to 80% plasma medium. The increase in caveolin mRNA levels and stimulation of FC efflux were reduced when LDL was removed by affinity chromatography from plasma in the extracellular medium. The induction of caveolin mRNA and FC efflux was also inhibited by cytochalasin D or hyperosmotic medium (Fig. 2A).
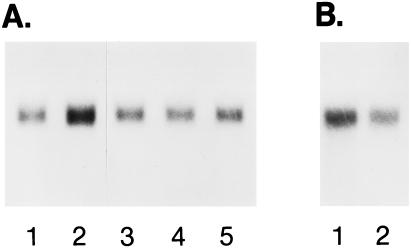
Figure 2.
(A) Effects of inhibitors of LDL–FC selective transfer on caveolin mRNA levels. Each lane contained 4 μg of total RNA. Caveolin mRNA levels represent the following: 1, baseline in 7% (vol/vol) plasma; 2, 80% (vol/vol) plasma without inhibitor (3 h, 37°C); 3, 80% (vol/vol) plasma in the presence of 40 μM cytochalasin D; 4, 80% (vol/vol) plasma in hyperosmotic PBS (22); and 5, 80% (vol/vol) plasma from which LDL had been selectively removed. Relative caveolin mRNA masses determined by densitometry, expressed relative to baseline in 7% (vol/vol) plasma medium, were 4.0, 1.2, 1.0, and 1.2 for lanes 2–5, respectively. Corresponding FC efflux rates (relative to baseline) were 3.9-, 1.7-, 1.5-, and 1.5-fold increased, respectively. (B) Effect of antisense DNA on caveolin mRNA levels after incubation with 50 μg of sense DNA (lane 1) or antisense DNA (lane 2). Each lane contained 4 μg of total RNA. FC efflux, determined under the conditions described in Fig. 1, was 38.9 ng·min−1 from sense-DNA-treated cells and 21.0 ng·min−1 from antisense-treated cells. mRNA in lane 2 was 0.58 relative to lane 1.
Effects of Caveolin Antisense DNA.
Studies described above showed an unexpected proportionality between caveolin mRNA levels and FC efflux. A further test of the relationship between FC transport and caveolin mRNA levels was made by selectively inhibiting caveolin mRNA synthesis with antisense DNA. Caveolin mRNA levels and FC efflux were measured in cells transfected with DNA sense or antisense to the 5′ end of the coding sequence of caveolin. There was a significant reduction (−42% ± 5%, four experiments) in the level of caveolin mRNA in these cells compared with cells without nucleotide or transfected with the same concentration of sense strand (Fig. 2B). Under the same conditions, FC efflux from 3H-FC labeled, antisense-treated cells was 20.4 ± 2.0 ng·min−1, compared with 37.5 ± 2.1 ng·min−1 for sense-treated cells, a mean decrease of −47% ± 4% (four experiments). These data suggest that FC efflux was limited by caveolin mRNA levels under these experimental conditions.
Regulation of Caveolar FC.
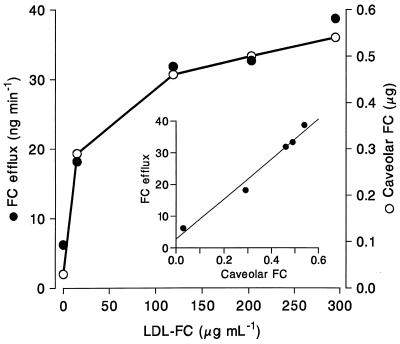
The relationship was determined between the mass of FC within the caveolae, assayed with cholesterol oxidase, and the rate at which FC effluxed to the medium under the same conditions. In view of evidence that LDL was a major source of FC transferred to the cells (2), the concentration of LDL in native plasma was varied by incorporating additional LDL purified by affinity chromatography into the extracellular medium. As LDL concentration increased, so did the mass of FC associated with caveolae, until saturation was approached at 2- to 3-fold native plasma LDL concentrations (Fig. 3). At all concentrations, the rate of FC efflux was proportional to caveolar FC mass.
Figure 3.
Effects of the LDL content of plasma on FC accessible to cholesterol oxidase at the cell surface, and on FC efflux. Cells equilibrated in 7% (vol/vol) 3H-FC labeled native plasma assayed directly, or transferred for 3 h at 37°C into 80% native plasma or plasma from which LDL had been removed by heparin affinity chromatography. In some dishes plasma LDL concentration was increased to ≈2- or 3-fold original levels by addition of LDL purified from the same 3H-FC-labeled plasma sample. After washing with unlabeled LDL, albumin, and PBS, caveolar FC was determined with cholesterol oxidase at 0–2°C; it is expressed as FC mass. FC efflux was determined after washing as the rate of transfer of cellular 3H-FC to unlabeled 80% plasma medium.
Effects of Oxysterols on Cellular FC Transport.
The effect of different oxysterols on the LDL–FC selective transfer pathway was determined. CαEP was incorporated into 3H-FC-labeled LDL as described in Experimental Methods, at a weight ratio (relative to FC) of 10%, comparable to conditions shown to inhibit FC efflux from peritoneal macrophages (17). No significant difference was detected in the rate at which FC from LDL with or without 10% CαEP was taken into the cells (33.5 ± 2.1 vs. 28.9 ± 3.6 ng·min−1, respectively). To study oxysterol effects on intracellular FC transport, cells were incubated at 15°C with 3H-FC-labeled LDL with or without 10% (wt/wt) CαEP. Under these conditions LDL-derived FC is transported through clathrin-coated pits to an intermediate density/trans-Golgi network (TGN) vesicle fraction, but not forwarded to the caveolae (21). When the cells were warmed (3 min, 37°C) to allow forward FC transport to the cell surface, a significant (≈50%) inhibition was observed in the 3H-FC appearing in the caveolae in the presence of oxysterol. There was a comparable decrease in FC efflux (Table 1). This indicates that the decrease in FC efflux observed in the presence of CαEP was the result of a defect, not in FC efflux from the caveolae, but in delivery of FC to the caveolae from the TGN.
Table 1.
FC transport to the cell surface in the presence and absence of CαEF
| Measurement | CαEP absent | CαEP present | Ratio |
|---|---|---|---|
| Caveolar FC,* ng | 7,120 ± 196 | 3,972 ± 391 | 0.56 ± 0.03 |
| FC efflux,† cpm | 4,490 ± 368 | 2,823 ± 220 | 0.62 ± 0.04 |
| Proportion of caveolar FC transferred to medium | 0.63 | 0.72 |
Values shown are means ± SD (n = 3).
Determined with cholesterol oxidase as cholest-4-en-3-one.
Determined over 3 min into unlabeled native plasma (80%, vol/vol) at 31°C. Monolayers had been prelabeled with 3H-FC LDL (58 μg·ml−1FC) ± 10% (wt/wt) CαEP for 60 min at 15°C to selectively label the intermediate density/TGN vesicle fraction (21). The cells were then brought to 31°C for 3 min prior to determination of caveolar FC label. Total cell-associated label was 15,162 ± 817 and 15,348 ± 1,724 cpm, respectively, for control and CαEP-modified LDL.
The effect of other oxysterols on intracellular FC transport was measured using similar techniques (Table 2). There was comparable inhibition in each case of the rate at which FC sensitive to cholesterol oxidase appeared at the cell surface.
Table 2.
Effects of oxysterols on the transport of FC to the cell surface
| Added sterol | Relative FC transport |
|---|---|
| FC | 0.99 ± 0.03 |
| CαEP | 0.41 ± 0.07 |
| CβEP | 0.65 ± 0.05 |
| 7K-C | 0.49 ± 0.06 |
| 7αOH-C | 0.59 ± 0.06 |
Fibroblast monolayers were preincubated with 3H-FC-labeled LDL enriched with FC [10% (wt/wt) relative to FC in native LDL] or the same proportion of oxysterol for 60 min at 15°C, then brought to 31°C for 3 min. 3H-FC accessible to cholesterol oxidase was measured over 4 h at 0–2°C. Values shown are means (±SD) relative to unmodified LDL.
Effects of Oxysterols on Caveolin mRNA Levels.
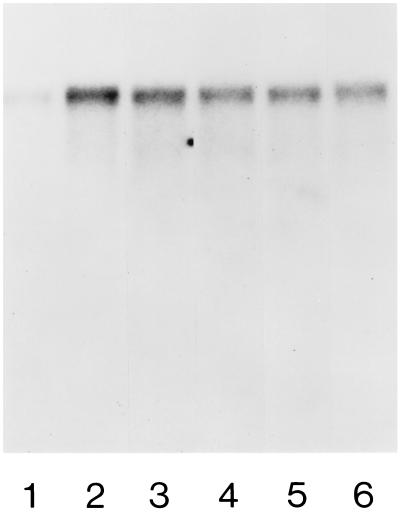
Caveolin mRNA levels are shown 3 h after the transfer of cells to 80% plasma medium/DMEM containing 10% (wt/wt) oxysterol relative to FC (Fig. 4). Each oxysterol reduced mRNA levels to a similar extent (≈50%) when present at 10% (wt/wt) relative to FC. These effects are comparable in magnitude to the reductions in FC transport and efflux described above.
Figure 4.
Effect of oxysterols on caveolin mRNA levels. Conditions are as described in the legend to Fig. 1. Cells equilibrated with 7% (vol/vol) plasma (lane 1) were transferred to medium containing 80% (vol/vol) plasma (lane 2); 80% (vol/vol) plasma + 10% (wt/wt) CαEP (lane 3); 80% plasma + 10% CβEP (lane 4); 80% plasma + 10% 7-KC (lane 5); or 80% plasma + 10% 7αOH-C (lane 6). Caveolin mRNA was increased 3.6-fold by 80% (vol/vol) plasma. mRNA levels were 0.46, 0.40, 0.36, and 0.34 of this level in the presence 10% (wt/wt) CαEP, CβEP, 7-KC, and 7αOH-FC, respectively. FC efflux rates were (3.9 ± 0.2)-fold increased by 80% (vol/vol) plasma, and were 0.54, 0.50, 0.46, and 0.46 of this increased level in the presence of the same oxysterols.
DISCUSSION
This study provides further evidence that FC efflux in quiescent fibroblast monolayers is dynamically regulated as part of cellular FC homeostasis. In these cells, the major external source of cellular FC was LDL, internalized by the selective transfer pathway. When the external concentration of LDL was increased, the rate of FC efflux adjusted so that cellular FC, although slightly increased, remained close to its original level (2). Data in the present study suggest that the increase in FC efflux responds to, and is triggered by, the increased uptake of LDL–FC. These experiments also show that FC efflux was directly proportional to FC mass in the cell surface caveolae, even though these microdomains make up only a small part (3–5%) of plasma membrane FC (4). FC in caveolae is transported from the intermediate density/TGN compartment by a pathway whose kinetic characteristics resemble those by which FC-sphingolipid rich “rafts” carrying newly synthesized proteins reach the cell surface (4, 11). The TGN, with the Golgi stacks, has been proposed to make up a standing FC gradient which sorts newly synthesized proteins into lysosome- and cell surface-directed traffic (29). On the basis of these concepts, we suggest that the selective FC transfer pathway described here and previously (2, 21) may form an integral part of the intracellular transport machinery for both proteins and FC. The caveolar or HDL-specific efflux pathway contributes about 50% of FC efflux in confluent fibroblast monolayers (22). These cells also express a “slow” or “nonspecific” component of efflux (22, 30). Whether FC for slow efflux originates in FC-glycolipid rafts unrecruited into caveolae, or by transfer from the cytofacial leaflet of the plasma membrane bilayer, presently remains unknown.
Caveolin is a key structural protein of caveolae. The present study shows that caveolin mRNA levels are up-regulated when increased FC enters the cell from LDL, probably mediated by the small increase in cellular FC under these conditions, which was observed earlier (2). The rate of FC efflux was up- or down-regulated in parallel with caveolin mRNA levels under several different conditions. Up-regulation occurred in the presence of purified LDL (when no medium HDL acceptor was present) to the same extent as in native plasma. This finding suggests that mRNA levels are responding to FC influx, and FC efflux follows from the increase in caveolin mRNA levels. Antisense DNA reduced both caveolin mRNA levels and FC efflux. These data are consistent with the suggestion that regulation of FC efflux by the caveolar pathway is mediated by caveolin mRNA.
7K-C is a major oxysterol of human atherosclerotic plaques (16, 31). It was recently identified as an inhibitor of FC efflux from macrophages (17). This observation has been confirmed and extended with 7-KC and several other oxysterols in confluent fibroblasts. Oxysterols did not affect the internalization of FC from LDL, but inhibited both the induction of caveolin mRNA and the transport of FC to the cell surface, in each case opposing the action of FC. The effectiveness of different oxysterols was generally similar in these studies. Concentrations of oxysterol required to halve FC efflux from fibroblasts were comparable to those shown to reduce efflux from peritoneal macrophages (17), but further research is needed to determine whether caveolin levels are reduced in plaques, and if this contributes to the accumulation of FC in lesions (31).
Modified sterols of several kinds have been reported to inhibit the intracellular transport of FC. Several plant sterols, oxysterols, and positively charged sterol analogs all induce FC accumulation intracellularly (32–34). The opposing effects of oxysterols and FC on caveolin mRNA levels and FC efflux found in this study could be explained if FC were transported from the intermediate density fraction/TGN to the endoplasmic reticulum prior to nucleoprotein-mediated regulation of caveolin mRNA levels. A mechanism of this kind has been proposed to explain the regulation of genes inhibited by FC (35), but it could apply equally to genes whose expression is up-regulated by FC, like caveolin. By this model, oxysterols would inhibit the transport of FC both from the TGN to the endoplasmic reticulum and between the TGN and the caveolae, a transfer upon which FC efflux depends (21). A better understanding of caveolin gene expression will be needed to determine if this hypothesis is correct. The caveolin genomic sequence has not yet been reported, nor have the contributions of promoter-dependent or other effects on caveolin mRNA levels been determined. Nevertheless, the present data provide strong support for the hypothesis that caveolin has a central role in cholesterol homeostasis in quiescent fibroblasts. The up-regulation of caveolin expression may represent a protective mechanism against cellular cholesterol accumulation in these cells.
Acknowledgments
We acknowledge the excellent technical assistance of Wilma Norona and Lolita Evangelista. This research was supported by the National Institutes of Health through Arteriosclerosis Specialized Center of Research HL 14237.
ABBREVIATIONS
- FC
free cholesterol
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- TGN
trans-Golgi network
- CαEP
cholesterol α-epoxide
- CβEP
cholesterol β-epoxide
- 7αOH-C
7α-hydroxycholesterol
- 7-KC
7-ketocholesterol
References
- 1.Goldstein J L, Brown M S, Anderson R G W, Russell D W, Schneider W J. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 2.Fielding C J, Fielding P E. Biochemistry. 1995;34:14237–14244. doi: 10.1021/bi00043a031. [DOI] [PubMed] [Google Scholar]
- 3.Fielding P E, Fielding C J. Biochemistry. 1995;34:14288–14292. doi: 10.1021/bi00044a004. [DOI] [PubMed] [Google Scholar]
- 4.Parton R G, Simons K. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- 5.Smart E J, Ying Y-S, Conrad P A, Anderson R G W. J Cell Biol. 1994;127:665–687. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parton R G, Joggerst B, Simons K. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnitzer J E, McIntosh D P, Dvorak A M, Liu J, Oh P. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 8.Murata M, Perannen J, Schreiner R, Wieland F, Purzchalia T V, Simons K. Proc Natl Acad Sci USA. 1995;92:339–343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Song K S, Lisanti M P. J Biol Chem. 1996;271:568–573. [PubMed] [Google Scholar]
- 10.Glenney J R. FEBS Lett. 1992;314:45–48. doi: 10.1016/0014-5793(92)81458-x. [DOI] [PubMed] [Google Scholar]
- 11.Fra A M, Williamson E, Simons K, Parton R G. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 12.Koleske A, Baltimore D, Lisanti M P. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fra A M, Williamson E, Simons K. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang W-J, Rothberg K G, Kamen B A, Anderson R G W. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berliner J A, Territo M C, Savanian A, Ramin S, Kim J, Bamshad B, Esterson M, Fogelman A M. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulten L M, Lindmark H, Diczfalusy U, Bjorkem I, Ottosson M, Liu Y, Bondjers G, Wiklund O. J Clin Invest. 1996;97:461–468. doi: 10.1172/JCI118436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelissen I C, Brown A J, Mander E L, Kritharides L, Dean R T, Jessup W. J Biol Chem. 1996;271:17852–17860. doi: 10.1074/jbc.271.30.17852. [DOI] [PubMed] [Google Scholar]
- 18.Heider J G, Boyett R L. J Lipid Res. 1978;19:514–518. [PubMed] [Google Scholar]
- 19.Miida T, Fielding C J, Fielding P E. Biochemistry. 1990;29:10469–10474. doi: 10.1021/bi00498a007. [DOI] [PubMed] [Google Scholar]
- 20.Fielding P E, Fielding C J. J Biol Chem. 1986;261:5233–5236. [PubMed] [Google Scholar]
- 21.Fielding P E, Fielding C J. Biochemistry. 1996;35:14932–14938. doi: 10.1021/bi9613382. [DOI] [PubMed] [Google Scholar]
- 22.Kawano M, Miida T, Fielding C J, Fielding P E. Biochemistry. 1993;32:5025–5028. doi: 10.1021/bi00070a008. [DOI] [PubMed] [Google Scholar]
- 23.Cupers P, Veither A, Kiss A, Baudhuin P, Courtnoy P J. J Cell Biol. 1994;127:725–735. doi: 10.1083/jcb.127.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glenney J R, Soppet D. Proc Natl Acad Sci USA. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurzchalia T V, Dupree P, Parton R G, Kellner R, Virta H, Lehnert M, Simons K. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnitzer J E, Liu J, Oh P. J Biol Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 28.Crompton M R, Owens R J, Totty N F, Moss S E, Waterfield M D, Crumpton M J. EMBO J. 1988;7:21–27. doi: 10.1002/j.1460-2075.1988.tb02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretscher M S, Munro S. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Czarnecka H, Yokoyama S. Biochim Biophys Acta. 1995;1259:227–234. doi: 10.1016/0005-2760(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 31.Suarna C, Dean R T, May J, Stocker R. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 32.Aviram M. J Biol Chem. 1992;267:218–225. [PubMed] [Google Scholar]
- 33.Sato Y, Nishikawa K, Aikawa K, Mimura K, Murakami-Murofushi K, Arai H, Inoue K. Biochim Biophys Acta. 1995;1257:38–46. doi: 10.1016/0005-2760(95)00053-f. [DOI] [PubMed] [Google Scholar]
- 34.Liscum L, Collins G J. J Biol Chem. 1991;266:16599–16606. [PubMed] [Google Scholar]
- 35.Lange Y, Steck T L. Trends Cell Biol. 1996;6:205–208. doi: 10.1016/0962-8924(96)20016-9. [DOI] [PubMed] [Google Scholar]