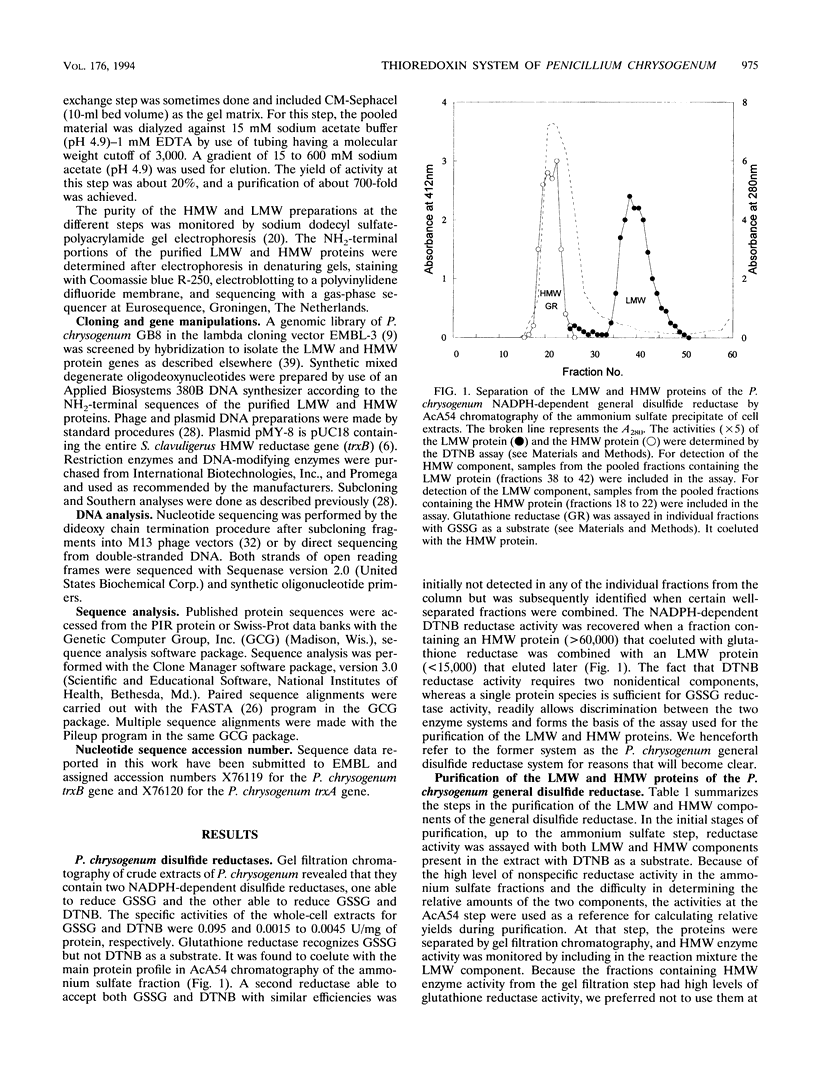
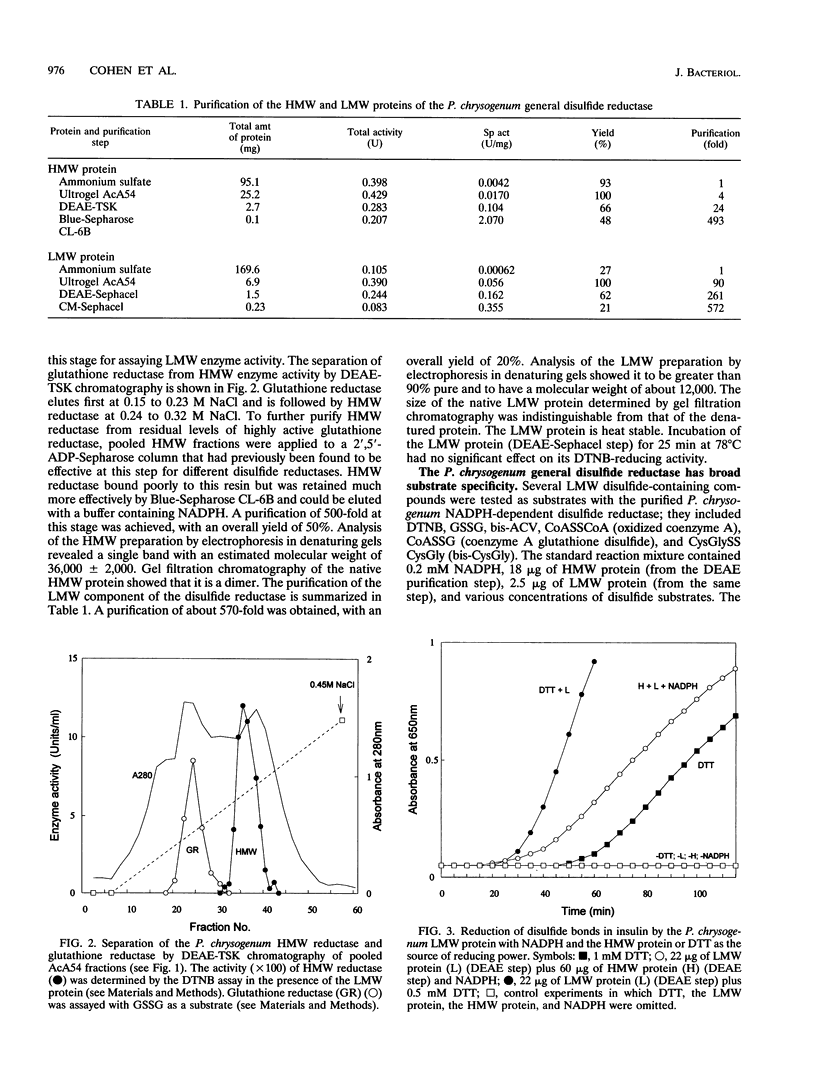
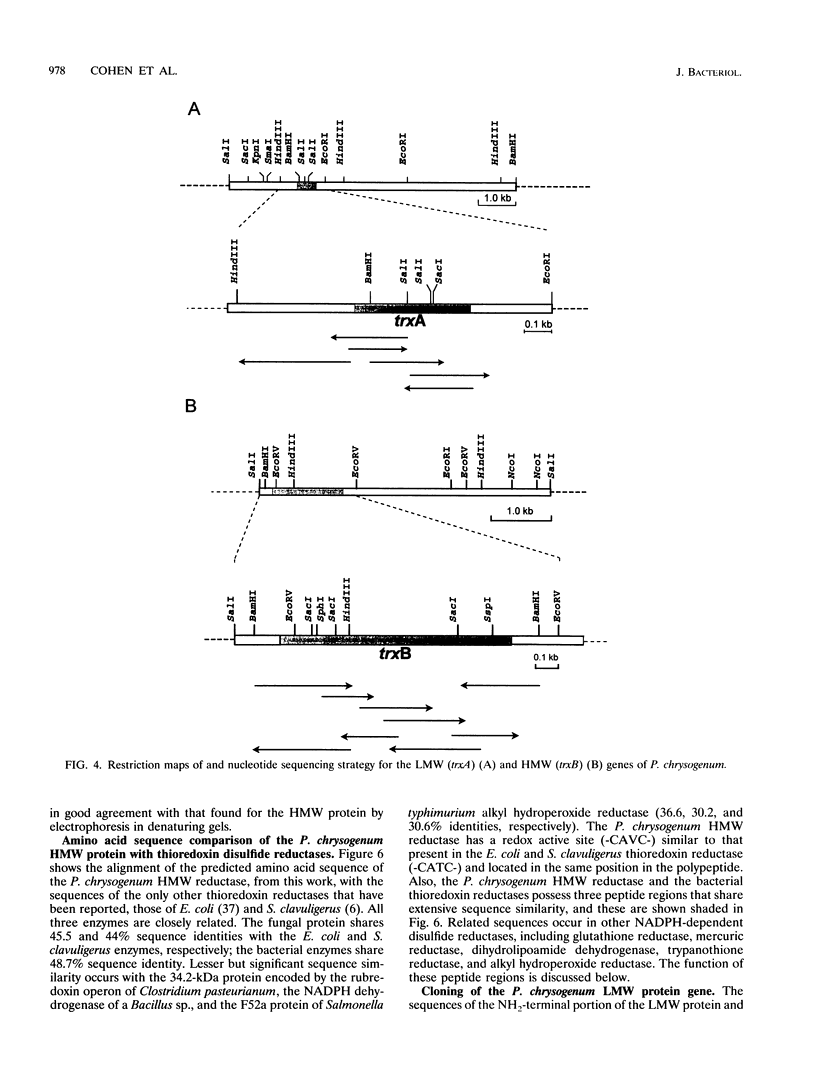
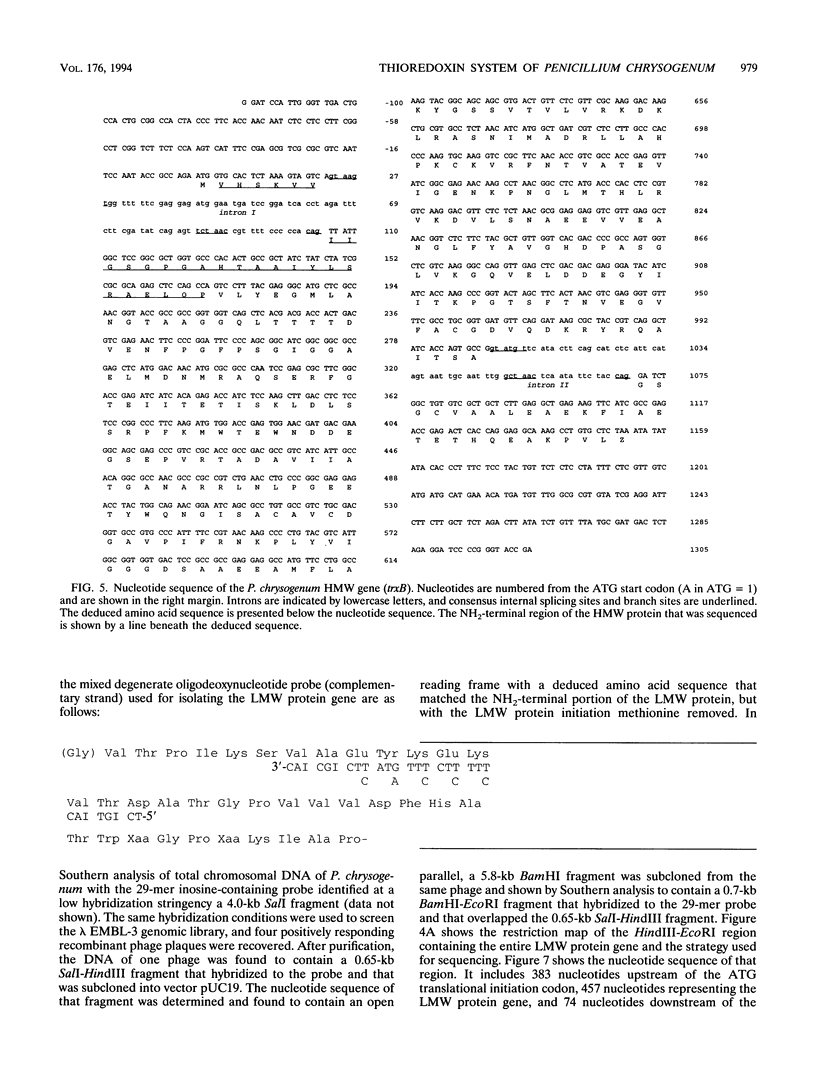
Abstract
Penicillium chrysogenum is an important producer of penicillin antibiotics. A key step in their biosynthesis is the oxidative cyclization of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine (ACV) to isopenicillin N by the enzyme isopenicillin N synthase (IPNS). bis-ACV, the oxidized disulfide form of ACV is, however, not a substrate for IPNS. We report here the characterization of a broad-range disulfide reductase from P. chrysogenum that efficiently reduces bis-ACV to the thiol monomer. When coupled in vitro with IPNS, it converts bis-ACV to isopenicillin N and may therefore play a role in penicillin biosynthesis. The disulfide reductase consists of two protein components, a 72-kDa NADPH-dependent reductase, containing two identical subunits, and a 12-kDa general disulfide reductant. The latter reduces disulfide bonds in low-molecular-weight compounds and in proteins. The genes coding for the reductase system were cloned and sequenced. Both possess introns. A comparative analysis of their predicted amino acid sequences showed that the 12-kDa protein shares 26 to 60% sequence identity with thioredoxins and that the 36-kDa protein subunit shares 44 to 49% sequence identity with the two known bacterial thioredoxin reductases. In addition, the P. chrysogenum NADPH-dependent reductase is able to accept thioredoxin as a substrate. These results establish that the P. chrysogenum broad-range disulfide reductase is a member of the thioredoxin family of oxidoreductases. This is the first example of the cloning of a eucaryotic thioredoxin reductase gene.
Full text
PDF




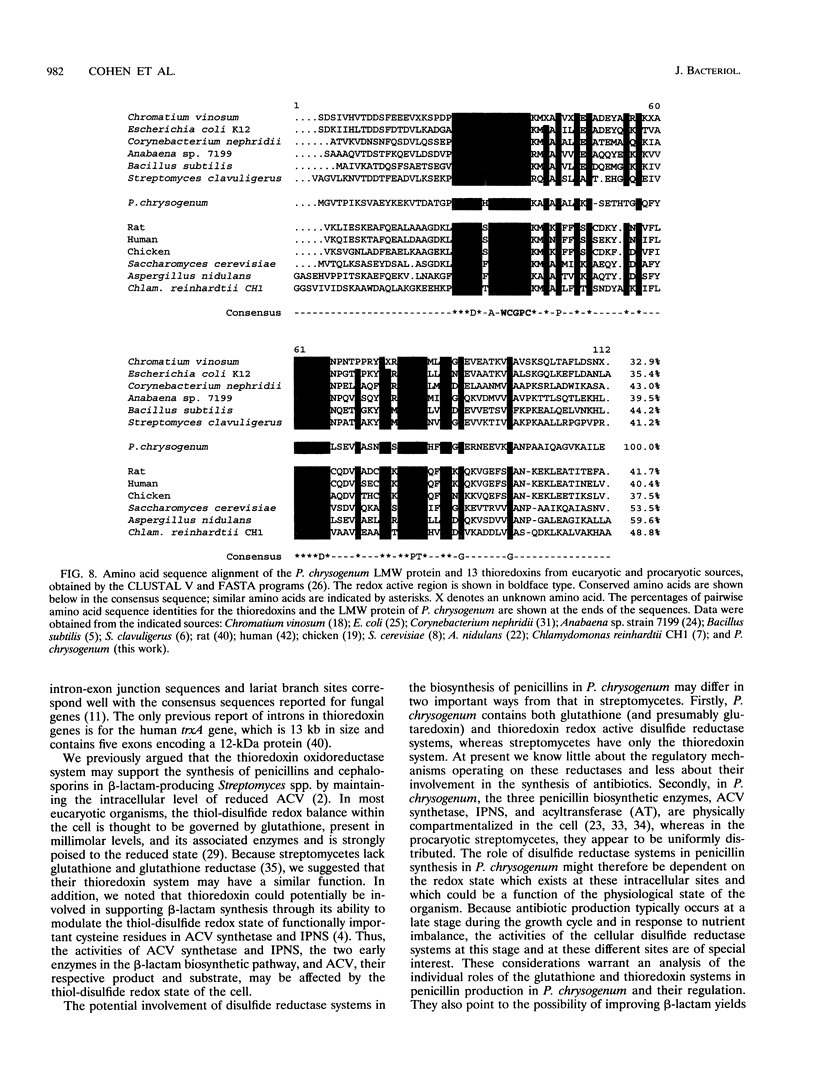


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboagye-Kwarteng T., Smith K., Fairlamb A. H. Molecular characterization of the trypanothione reductase gene from Crithidia fasciculata and Trypanosoma brucei: comparison with other flavoprotein disulphide oxidoreductases with respect to substrate specificity and catalytic mechanism. Mol Microbiol. 1992 Nov;6(21):3089–3099. doi: 10.1111/j.1365-2958.1992.tb01766.x. [DOI] [PubMed] [Google Scholar]
- Aharonowitz Y., Av-Gay Y., Schreiber R., Cohen G. Characterization of a broad-range disulfide reductase from Streptomyces clavuligerus and its possible role in beta-lactam antibiotic biosynthesis. J Bacteriol. 1993 Feb;175(3):623–629. doi: 10.1128/jb.175.3.623-629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonowitz Y., Cohen G., Martin J. F. Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation, and evolution. Annu Rev Microbiol. 1992;46:461–495. doi: 10.1146/annurev.mi.46.100192.002333. [DOI] [PubMed] [Google Scholar]
- Chen N. Y., Zhang J. J., Paulus H. Chromosomal location of the Bacillus subtilis aspartokinase II gene and nucleotide sequence of the adjacent genes homologous to uvrC and trx of Escherichia coli. J Gen Microbiol. 1989 Nov;135(11):2931–2940. doi: 10.1099/00221287-135-11-2931. [DOI] [PubMed] [Google Scholar]
- Cohen G., Yanko M., Mislovati M., Argaman A., Schreiber R., Av-Gay Y., Aharonowitz Y. Thioredoxin-thioredoxin reductase system of Streptomyces clavuligerus: sequences, expression, and organization of the genes. J Bacteriol. 1993 Aug;175(16):5159–5167. doi: 10.1128/jb.175.16.5159-5167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decottignies P., Schmitter J. M., Dutka S., Jacquot J. P., Miginiac-Maslow M. Characterization and primary structure of a second thioredoxin from the green alga, Chlamydomonas reinhardtii. Eur J Biochem. 1991 Jun 1;198(2):505–512. doi: 10.1111/j.1432-1033.1991.tb16043.x. [DOI] [PubMed] [Google Scholar]
- Gan Z. R. Yeast thioredoxin genes. J Biol Chem. 1991 Jan 25;266(3):1692–1696. [PubMed] [Google Scholar]
- Gouka R. J., van Hartingsveldt W., Bovenberg R. A., van den Hondel C. A., van Gorcom R. F. Cloning of the nitrate-nitrite reductase gene cluster of Penicillium chrysogenum and use of the niaD gene as a homologous selection marker. J Biotechnol. 1991 Sep;20(2):189–199. doi: 10.1016/0168-1656(91)90227-m. [DOI] [PubMed] [Google Scholar]
- Gravina S. A., Mieyal J. J. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993 Apr 6;32(13):3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- Haller B. L., Fuchs J. A. Mapping of trxB, a mutation responsible for reduced thioredoxin reductase activity. J Bacteriol. 1984 Sep;159(3):1060–1062. doi: 10.1128/jb.159.3.1060-1062.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Leskiw B. K., Vining L. C., Aharonowitz Y., Westlake D. W., Wolfe S. Purification of isopenicillin N synthetase from Streptomyces clavuligerus. Can J Microbiol. 1986 Dec;32(12):953–958. doi: 10.1139/m86-176. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Biemann K. The primary structure of thioredoxin from Chromatium vinosum determined by high-performance tandem mass spectrometry. Biochemistry. 1987 Mar 10;26(5):1209–1214. doi: 10.1021/bi00379a001. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Luk K. C. Isolation of a chicken thioredoxin cDNA clone. Thioredoxin mRNA is differentially expressed in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. J Biol Chem. 1988 Jul 15;263(20):9607–9611. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landman O., Shiffman D., Av-Gay Y., Aharonowitz Y., Cohen G. High level expression in Escherichia coli of isopenicillin N synthase genes from Flavobacterium and Streptomyces, and recovery of active enzyme from inclusion bodies. FEMS Microbiol Lett. 1991 Dec 1;68(3):239–244. doi: 10.1016/0378-1097(91)90362-e. [DOI] [PubMed] [Google Scholar]
- Le Marechal P., Hoang B. M., Schmitter J. M., Van Dorsselaer A., Decottignies P. Purification, properties and primary structure of thioredoxin from Aspergillus nidulans. Eur J Biochem. 1992 Dec 1;210(2):421–429. doi: 10.1111/j.1432-1033.1992.tb17437.x. [DOI] [PubMed] [Google Scholar]
- Lendenfeld T., Ghali D., Wolschek M., Kubicek-Pranz E. M., Kubicek C. P. Subcellular compartmentation of penicillin biosynthesis in Penicillium chrysogenum. The amino acid precursors are derived from the vacuole. J Biol Chem. 1993 Jan 5;268(1):665–671. [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Gleason F. K., Fuchs J. A. Cloning, expression, and characterization of the Anabaena thioredoxin gene in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1258–1264. doi: 10.1128/jb.168.3.1258-1264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Luthman M., Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982 Dec 21;21(26):6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Chase J. W., Richardson C. C. Genetic mapping of trxA, a gene affecting thioredoxin in Escherichia coli K12. Mol Gen Genet. 1977 Oct 20;155(2):145–152. doi: 10.1007/BF00393153. [DOI] [PubMed] [Google Scholar]
- Meng M., Hogenkamp H. P. Purification, characterization, and amino acid sequence of thioredoxin from Corynebacterium nephridii. J Biol Chem. 1981 Sep 10;256(17):9174–9182. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Müller W. H., Bovenberg R. A., Groothuis M. H., Kattevilder F., Smaal E. B., Van der Voort L. H., Verkleij A. J. Involvement of microbodies in penicillin biosynthesis. Biochim Biophys Acta. 1992 Apr 22;1116(2):210–213. doi: 10.1016/0304-4165(92)90118-e. [DOI] [PubMed] [Google Scholar]
- Müller W. H., van der Krift T. P., Krouwer A. J., Wösten H. A., van der Voort L. H., Smaal E. B., Verkleij A. J. Localization of the pathway of the penicillin biosynthesis in Penicillium chrysogenum. EMBO J. 1991 Feb;10(2):489–495. doi: 10.1002/j.1460-2075.1991.tb07971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton G. L., Fahey R. C., Cohen G., Aharonowitz Y. Low-molecular-weight thiols in streptomycetes and their potential role as antioxidants. J Bacteriol. 1993 May;175(9):2734–2742. doi: 10.1128/jb.175.9.2734-2742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Abraham E. P., Baldwin J. E. Factors affecting the isopenicillin N synthetase reaction. Biochem J. 1988 Oct 1;255(1):345–351. [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- Tonissen K. F., Wells J. R. Isolation and characterization of human thioredoxin-encoding genes. Gene. 1991 Jun 30;102(2):221–228. doi: 10.1016/0378-1119(91)90081-l. [DOI] [PubMed] [Google Scholar]
- Wolfe S., Demain A. L., Jensen S. E., Westlake D. W. Enzymatic approach to syntheses of unnatural beta-lactams. Science. 1984 Dec 21;226(4681):1386–1392. doi: 10.1126/science.6390683. [DOI] [PubMed] [Google Scholar]
- Wollman E. E., d'Auriol L., Rimsky L., Shaw A., Jacquot J. P., Wingfield P., Graber P., Dessarps F., Robin P., Galibert F. Cloning and expression of a cDNA for human thioredoxin. J Biol Chem. 1988 Oct 25;263(30):15506–15512. [PubMed] [Google Scholar]





