Abstract
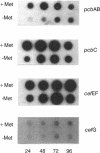
Methionine stimulated cephalosporin production in cultures of three different strains of Acremonium chrysogenum when added either at inoculation time or at 72 h to cells grown previously in the absence of methionine. When methionine was added at 72 h, the stimulation of cephalosporin biosynthesis was observed only 12 h later and required de novo protein synthesis. Methionine increased the levels of enzymes (isopenicillin N synthase and deacetylcephalosporin C acetyltransferase) expressed from genes (pcbC and cefG, respectively) located in the two clusters of cephalosporin biosynthesis genes in the wild-type A. chrysogenum strain and also in the two improved strains, CW19 and C10. Methionine-supplemented cells showed higher levels of transcripts of the four known genes (pcbAB, pcbC, cefEF and, to a slight extent, cefG) of the cephalosporin biosynthetic pathway than cells grown in the absence of methionine. The levels of the cefG transcript were much lower than those of the pcbAB, pcbC, and cefEF transcripts. The induction by methionine of transcription of the four cephalosporin biosynthesis genes and the known effect of this amino acid on the differentiation of A. chrysogenum indicate that methionine exerts a pleiotropic effect that coordinately regulates cephalosporin biosynthesis and differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. E., Masison D. C. Isolation of the gene encoding the Saccharomyces cerevisiae centromere-binding protein CP1. Mol Cell Biol. 1990 Jun;10(6):2458–2467. doi: 10.1128/mcb.10.6.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J. L., Cantoral J. M., Alvarez E., Díez B., Martín J. F. Cloning, sequence analysis and transcriptional study of the isopenicillin N synthase of Penicillium chrysogenum AS-P-78. Mol Gen Genet. 1989 Mar;216(1):91–98. doi: 10.1007/BF00332235. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai M., Davis R. W. Yeast centromere binding protein CBF1, of the helix-loop-helix protein family, is required for chromosome stability and methionine prototrophy. Cell. 1990 May 4;61(3):437–446. doi: 10.1016/0092-8674(90)90525-j. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Winstanley D. J., Demain A. L. Effect of norleucine on mycelial fragmentation in Cephalosporium acremonium. Appl Environ Microbiol. 1976 Jan;31(1):143–145. doi: 10.1128/aem.31.1.143-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez S., Díez B., Montenegro E., Martín J. F. Characterization of the Cephalosporium acremonium pcbAB gene encoding alpha-aminoadipyl-cysteinyl-valine synthetase, a large multidomain peptide synthetase: linkage to the pcbC gene as a cluster of early cephalosporin biosynthetic genes and evidence of multiple functional domains. J Bacteriol. 1991 Apr;173(7):2354–2365. doi: 10.1128/jb.173.7.2354-2365.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez S., Velasco J., Fernandez F. J., Martín J. F. The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J Bacteriol. 1992 May;174(9):3056–3064. doi: 10.1128/jb.174.9.3056-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K. I., Mizuno M., Kodaira R. Effect of methionine on cephalosporin C and penicillin N production by a mutant of Cephalosporium acremonium. J Antibiot (Tokyo) 1975 Nov;28(11):881–888. doi: 10.7164/antibiotics.28.881. [DOI] [PubMed] [Google Scholar]
- Korch C., Mountain H. A., Byström A. S. Cloning, nucleotide sequence, and regulation of MET14, the gene encoding the APS kinase of Saccharomyces cerevisiae. Mol Gen Genet. 1991 Sep;229(1):96–108. doi: 10.1007/BF00264218. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D. M., Martín J. F. Carbon catabolite regulation of the conversion of penicillin N into cephalosporin C. J Antibiot (Tokyo) 1983 Jun;36(6):700–708. doi: 10.7164/antibiotics.36.700. [DOI] [PubMed] [Google Scholar]
- Martin J. F. Clusters of genes for the biosynthesis of antibiotics: regulatory genes and overproduction of pharmaceuticals. J Ind Microbiol. 1992 Feb-Mar;9(2):73–90. doi: 10.1007/BF01569737. [DOI] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- Mellor J., Jiang W., Funk M., Rathjen J., Barnes C. A., Hinz T., Hegemann J. H., Philippsen P. CPF1, a yeast protein which functions in centromeres and promoters. EMBO J. 1990 Dec;9(12):4017–4026. doi: 10.1002/j.1460-2075.1990.tb07623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Huber F. M. Antibiotic synthesis and morphological differentiation of Cephalosporium acremonium. Appl Microbiol. 1971 Jul;22(1):6–10. doi: 10.1128/am.22.1.6-10.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüesch J., Heim J., Treichler H. J. The biosynthesis of sulfur-containing beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:51–75. doi: 10.1146/annurev.mi.41.100187.000411. [DOI] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M., McQuade B. A., Saunders K., Turner M. K., Harford S. Characterization of a loss-of-function mutation in the isopenicillin N synthetase gene of Acremonium chrysogenum. Gene. 1989 Dec 21;85(1):267–273. doi: 10.1016/0378-1119(89)90493-9. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Skatrud P. L., Queener S. W. An electrophoretic molecular karyotype for an industrial strain of Cephalosporium acremonium. Gene. 1989 May 30;78(2):331–338. doi: 10.1016/0378-1119(89)90235-7. [DOI] [PubMed] [Google Scholar]
- Thomas D., Cherest H., Surdin-Kerjan Y. Elements involved in S-adenosylmethionine-mediated regulation of the Saccharomyces cerevisiae MET25 gene. Mol Cell Biol. 1989 Aug;9(8):3292–3298. doi: 10.1128/mcb.9.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vian A., Peñalva M. A. Cloning of the pyr4 gene encoding orotidine-5'-phosphate decarboxylase in Cephalosporium acremonium. Curr Genet. 1990 Mar;17(3):223–227. doi: 10.1007/BF00312613. [DOI] [PubMed] [Google Scholar]
- Vogel K., Hörz W., Hinnen A. The two positively acting regulatory proteins PHO2 and PHO4 physically interact with PHO5 upstream activation regions. Mol Cell Biol. 1989 May;9(5):2050–2057. doi: 10.1128/mcb.9.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]