Abstract
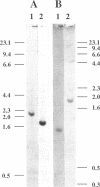
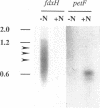
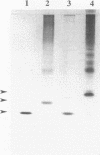
A genomic DNA region with four consecutive open reading frames, including an fdxH-type gene, has been sequenced and initially characterized for the nonheterocystous nitrogen-fixing cyanobacterium Plectonema boryanum PCC 73110. The fdxH gene encodes a [2Fe-2S]-type ferredoxin, 98 amino acids in length, with a deduced molecular mass of 10.9 kDa. Conserved residues include two characteristic lysines at positions 10 and 11, shown recently to be important for interaction with nitrogenase reductase (S. Schmitz, B. Schrautermeier, and H. Böhme, Mol. Gen. Genet. 240:455-460, 1993). The gene is transcribed only under anaerobic nitrogenase-inducing conditions, whereas the Plectonema petF gene, encoding a different (type 1) [2Fe-2S] ferredoxin, is only transcribed in cultures growing with combined nitrogen. The fdxH gene was expressed in Escherichia coli as a holoprotein. The purified protein was able to effectively donate electrons to cyanobacterial nitrogenase, whereas PetF from the same organism was not. The occurrence of FdxH in the nonheterocystous genus Plectonema demonstrates for the first time that FdxH-type ferredoxins are not exclusively expressed within heterocysts, as is true for cyanobacteria differentiating these cells for nitrogen fixation under aerobic growth conditions. Two open reading frames that precede fdxH have high similarity to those found at a corresponding location in Anabaena sp. strain PCC 7120. In the latter organism, they are transcribed only under nitrogen-fixing conditions, but the functions of their gene products remain unclear (D. Borthakur, M. Basche, W. J. Buikema, P. B. Borthakur, and R. Haselkorn, Mol. Gen. Genet. 221:227-234, 1990). An fdxB-type gene encoding a 2[4Fe-4S] ferredoxin not previously identified in cyanobacteria is located immediately downstream of fdxH in P. boryanum.
Full text
PDF

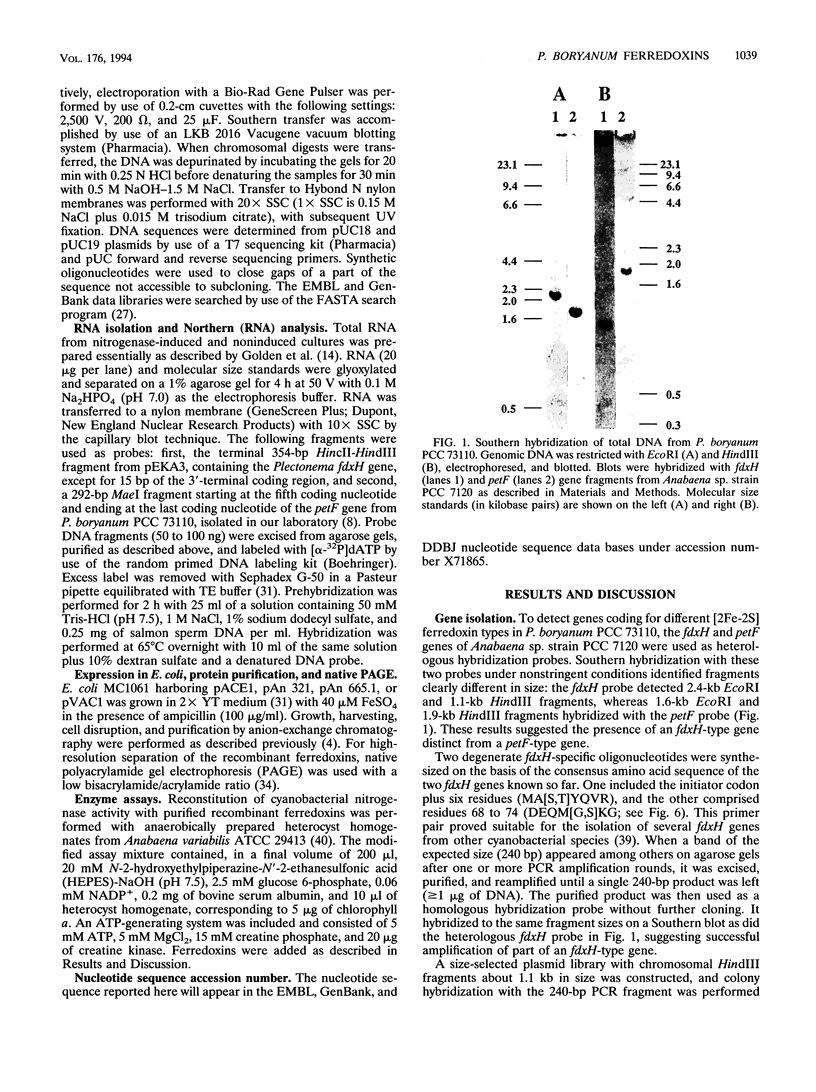






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Whitaker R. A., Krogmann D. W., Curtis S. E. Isolation and sequence of the gene for ferredoxin I from the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1986 Dec;168(3):1265–1271. doi: 10.1128/jb.168.3.1265-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. The discovery of ferredoxin: the photosynthetic path. Trends Biochem Sci. 1988 Jan;13(1):30–33. doi: 10.1016/0968-0004(88)90016-3. [DOI] [PubMed] [Google Scholar]
- Borthakur D., Basche M., Buikema W. J., Borthakur P. B., Haselkorn R. Expression, nucleotide sequence and mutational analysis of two open reading frames in the nif gene region of Anabaena sp. strain PCC7120. Mol Gen Genet. 1990 Apr;221(2):227–234. doi: 10.1007/BF00261725. [DOI] [PubMed] [Google Scholar]
- Böhme H., Haselkorn R. Molecular cloning and nucleotide sequence analysis of the gene coding for heterocyst ferredoxin from the cyanobacterium Anabaena sp. strain PCC 7120. Mol Gen Genet. 1988 Oct;214(2):278–285. doi: 10.1007/BF00337722. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Charng Y. Y., Kakefuda G., Iglesias A. A., Buikema W. J., Preiss J. Molecular cloning and expression of the gene encoding ADP-glucose pyrophosphorylase from the cyanobacterium Anabaena sp. strain PCC 7120. Plant Mol Biol. 1992 Oct;20(1):37–47. doi: 10.1007/BF00029147. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Hurley J. K., Salamon Z., Meyer T. E., Fitch J. C., Cusanovich M. A., Markley J. L., Cheng H., Xia B., Chae Y. K., Medina M. Amino acid residues in Anabaena ferredoxin crucial to interaction with ferredoxin-NADP+ reductase: site-directed mutagenesis and laser flash photolysis. Biochemistry. 1993 Sep 14;32(36):9346–9354. doi: 10.1021/bi00087a013. [DOI] [PubMed] [Google Scholar]
- Jacobson B. L., Chae Y. K., Markley J. L., Rayment I., Holden H. M. Molecular structure of the oxidized, recombinant, heterocyst [2Fe-2S] ferredoxin from Anabaena 7120 determined to 1.7-A resolution. Biochemistry. 1993 Jul 6;32(26):6788–6793. doi: 10.1021/bi00077a033. [DOI] [PubMed] [Google Scholar]
- Jouanneau Y., Meyer C., Gaillard J., Forest E., Gagnon J. Purification and characterization of a novel dimeric ferredoxin (FdIII) from Rhodobacter capsulatus. J Biol Chem. 1993 May 15;268(14):10636–10644. [PubMed] [Google Scholar]
- Kakefuda G., Charng Y. Y., Iglesias A. A., McIntosh L., Preiss J. Molecular Cloning and Sequencing of ADP-Glucose Pyrophosphorylase from Synechocystis PCC 6803. Plant Physiol. 1992 May;99(1):359–361. doi: 10.1104/pp.99.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus P. A., Daniels M. J., Herriott J. R. Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science. 1991 Jan 4;251(4989):60–66. [PubMed] [Google Scholar]
- Klipp W., Reiländer H., Schlüter A., Krey R., Pühler A. The Rhizobium meliloti fdxN gene encoding a ferredoxin-like protein is necessary for nitrogen fixation and is cotranscribed with nifA and nifB. Mol Gen Genet. 1989 Apr;216(2-3):293–302. doi: 10.1007/BF00334368. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Hirasawa M. Ferredoxin-dependent chloroplast enzymes. Biochim Biophys Acta. 1991 Jan 22;1056(2):93–125. doi: 10.1016/s0005-2728(05)80277-4. [DOI] [PubMed] [Google Scholar]
- Lomax T. L., Conley P. B., Schilling J., Grossman A. R. Isolation and characterization of light-regulated phycobilisome linker polypeptide genes and their transcription as a polycistronic mRNA. J Bacteriol. 1987 Jun;169(6):2675–2684. doi: 10.1128/jb.169.6.2675-2684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Vivian C., Hennecke S., Pühler A., Klipp W. Open reading frame 5 (ORF5), encoding a ferredoxinlike protein, and nifQ are cotranscribed with nifE, nifN, nifX, and ORF4 in Rhodobacter capsulatus. J Bacteriol. 1989 May;171(5):2591–2598. doi: 10.1128/jb.171.5.2591-2598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Buikema W. J., Haselkorn R. Bacterial-type ferredoxin genes in the nitrogen fixation regions of the cyanobacterium Anabaena sp. strain PCC 7120 and Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4406–4410. doi: 10.1128/jb.170.9.4406-4410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K., Suetsugu Y., Tokuda K., Miyatake Y., Young D. A., Marrs B. L., Matsubara H. Genetic analysis of functional differences among distinct ferredoxins in Rhodobacter capsulatus. J Biol Chem. 1991 Jul 15;266(20):12889–12895. [PubMed] [Google Scholar]
- Schatt E., Jouanneau Y., Vignais P. M. Molecular cloning and sequence analysis of the structural gene of ferredoxin I from the photosynthetic bacterium Rhodobacter capsulatus. J Bacteriol. 1989 Nov;171(11):6218–6226. doi: 10.1128/jb.171.11.6218-6226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz S., Schrautemeier B., Böhme H. Evidence from directed mutagenesis that positively charged amino acids are necessary for interaction of nitrogenase with the [2Fe-2S] heterocyst ferredoxin (FdxH) from the cyanobacterium Anabaena sp., PCC7120. Mol Gen Genet. 1993 Sep;240(3):455–460. doi: 10.1007/BF00280401. [DOI] [PubMed] [Google Scholar]
- Suzuki J. Y., Bauer C. E. Light-independent chlorophyll biosynthesis: involvement of the chloroplast gene chlL (frxC). Plant Cell. 1992 Aug;4(8):929–940. doi: 10.1105/tpc.4.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel T. Characterization of genes for an alternative nitrogenase in the cyanobacterium Anabaena variabilis. J Bacteriol. 1993 Oct;175(19):6276–6286. doi: 10.1128/jb.175.19.6276-6286.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare N. M., Benemann J. R. Nitrogenase activity and photosynthesis in Plectonema boryanum. J Bacteriol. 1974 Jul;119(1):258–265. doi: 10.1128/jb.119.1.258-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunin A. F., Hallenbeck P. C., Troshina OYu, Gogotov I. N. Purification and properties of a bacterial-type ferredoxin from the nitrogen-fixing cyanobacterium Anabaena variabilis ATCC29413. Biochim Biophys Acta. 1993 May 13;1163(2):124–130. doi: 10.1016/0167-4838(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]