Abstract
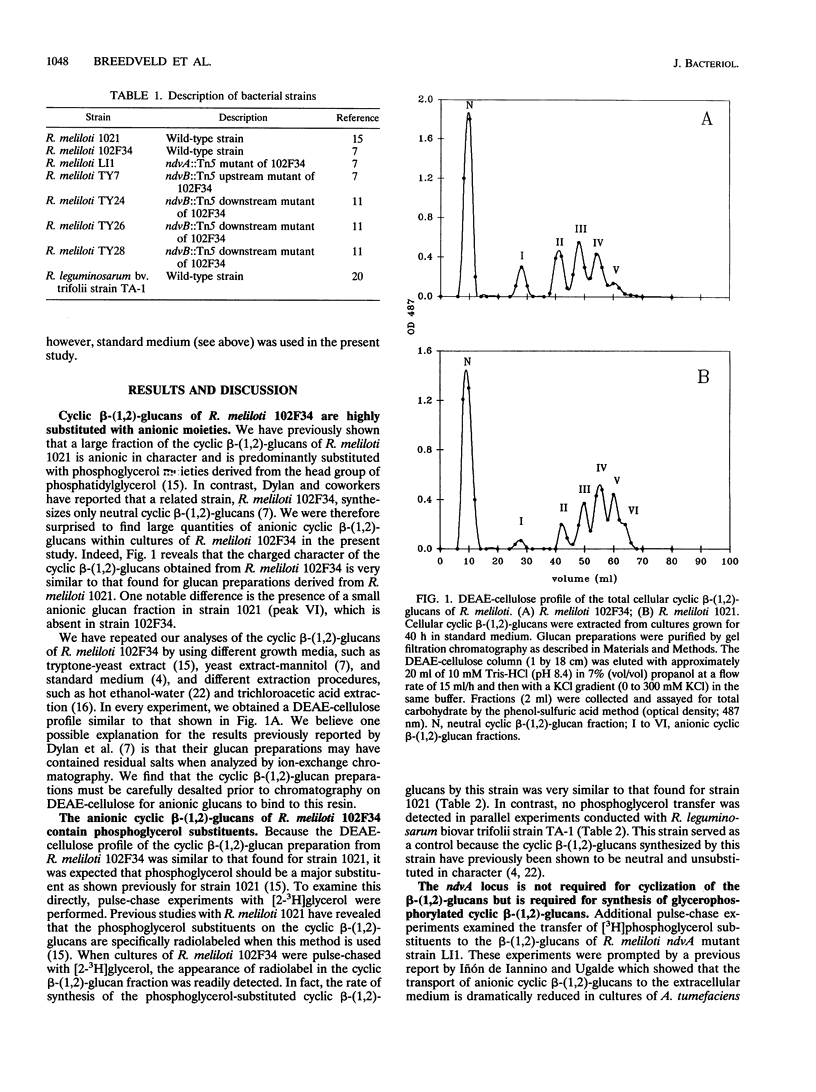
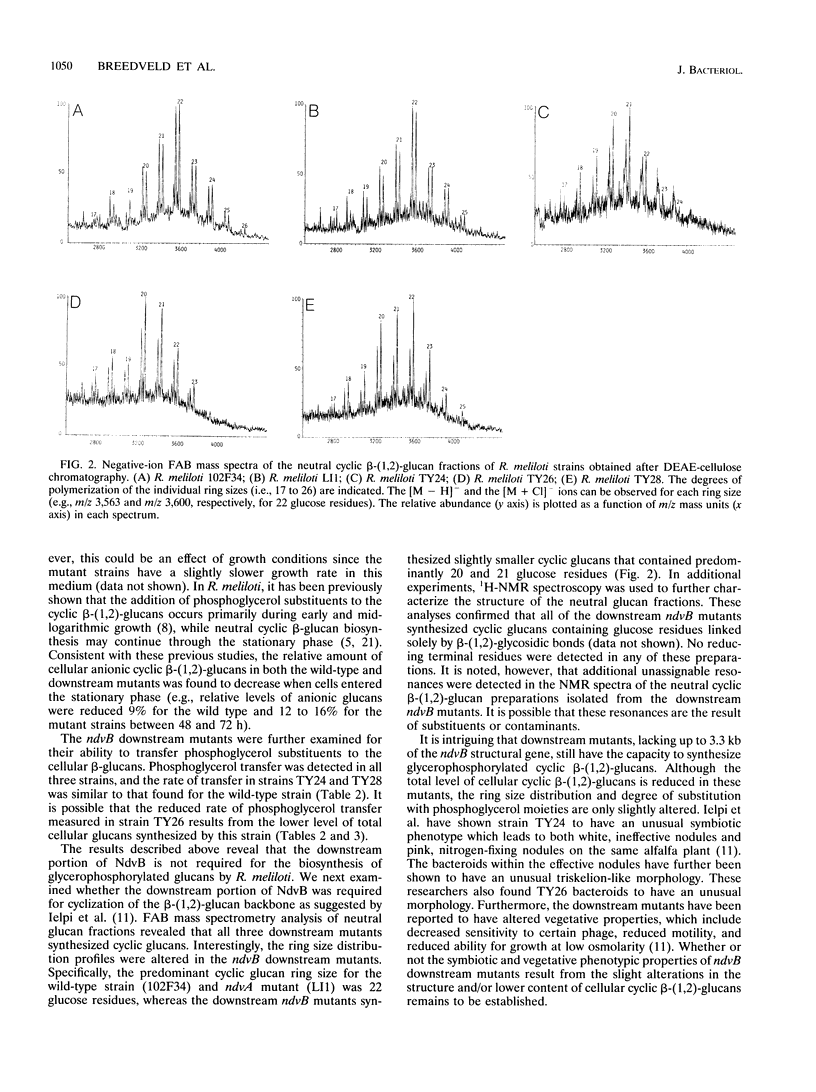
The periplasmic cyclic beta-(1,2)-glucans of Rhizobium spp. are believed to provide functions during hypoosmotic adaptation and legume nodulation. In Rhizobium meliloti, cyclic beta-(1,2)-glucans are synthesized at highest levels when cells are grown at low osmolarity, and a considerable fraction (> or = 35%) of these glucans may become substituted with phosphoglycerol moieties. Thus far, two chromosomally encoded proteins, NdvA and NdvB, have been shown to function during cyclic beta-(1,2)-glucan biosynthesis; however, the precise roles for these proteins remain unclear. In the present study, we show that R. meliloti mutants lacking up to one-third of the downstream region of ndvB synthesize cyclic beta-(1,2)-glucans similar to those produced by wild-type cells with respect to size and phosphoglycerol substituent profile. In contrast, no phosphoglycerol substituents were detected on the cyclic beta-(1,2)-glucans synthesized by an R. meliloti ndvA mutant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breedveld M. W., Zevenhuizen L. P., Zehnder A. J. Excessive excretion of cyclic beta-(1,2)-glucan by Rhizobium trifolii TA-1. Appl Environ Microbiol. 1990 Jul;56(7):2080–2086. doi: 10.1128/aem.56.7.2080-2086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Martinetti G., Leigh J. A., Lee C. C., Thienes C., Theines C., Nester E. W. Role for [corrected] Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J Bacteriol. 1989 Mar;171(3):1609–1615. doi: 10.1128/jb.171.3.1609-1615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger O., Weissborn A. C., Kennedy E. P. Biosynthesis and excretion of cyclic glucans by Rhizobium meliloti 1021. J Bacteriol. 1991 May;173(9):3021–3024. doi: 10.1128/jb.173.9.3021-3024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Rumley M. K., Kennedy E. P. Biosynthesis of membrane-derived oligosaccharides: a periplasmic phosphoglyceroltransferase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5513–5517. doi: 10.1073/pnas.78.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu M. Cyclic (1----2)-beta-D-glucans (cyclosophorans) produced by Agrobacterium and Rhizobium species. Carbohydr Res. 1992 Jul 2;231:137–146. doi: 10.1016/0008-6215(92)84014-j. [DOI] [PubMed] [Google Scholar]
- Ielpi L., Dylan T., Ditta G. S., Helinski D. R., Stanfield S. W. The ndvB locus of Rhizobium meliloti encodes a 319-kDa protein involved in the production of beta-(1----2)-glucan. J Biol Chem. 1990 Feb 15;265(5):2843–2851. [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Benesi A. J. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J Bacteriol. 1988 Oct;170(10):4569–4575. doi: 10.1128/jb.170.10.4569-4575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- O'Connell K. P., Handelsman J. chvA locus may be involved in export of neutral cyclic beta-1,2-linked D-glucan from Agrobacterium tumefaciens. Mol Plant Microbe Interact. 1989 Jan-Feb;2(1):11–16. [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Igari D., Tanahashi Y., Harada K., Saito M. Measurements of size and weight of prostate by means of transrectal ultrasonotomography. Tohoku J Exp Med. 1974 Nov;114(3):277–285. doi: 10.1620/tjem.114.277. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P., van Veldhuizen A., Fokkens R. H. Re-examination of cellular cyclic beta-1,2-glucans of Rhizobiaceae: distribution of ring sizes and degrees of glycerol-1-phosphate substitution. Antonie Van Leeuwenhoek. 1990 Apr;57(3):173–178. doi: 10.1007/BF00403952. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Geremia R. A., Cavaignac S., Cangelosi G. A., Nester E. W., Ugalde R. A. Identification of the product of an Agrobacterium tumefaciens chromosomal virulence gene. Mol Plant Microbe Interact. 1988 Mar;1(3):121–127. doi: 10.1094/mpmi-1-121. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Ugalde R. A. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in beta-D(1-2) glucan synthesis. J Bacteriol. 1986 Sep;167(3):947–951. doi: 10.1128/jb.167.3.947-951.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino N. I., Ugalde R. A. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1-2)glucan. J Bacteriol. 1989 May;171(5):2842–2849. doi: 10.1128/jb.171.5.2842-2849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


