Abstract
In kidney epithelial cells, arachidonic acid and other fatty acids are important signal transduction molecules for G protein-coupled receptors. We now demonstrate that arachidonic acid induced a time- and dose-dependent activation of JNK, a member of the mitogen-activated protein kinase family, as assessed by phosphorylation of the transcription factor ATF-2. Increments in JNK activity were detectable at 5 μM arachidonic acid and plateaued at 30 μM. Activation was specific to arachidonic acid and linoleic acid, since other fatty acids of the n − 3 and n − 6 series and/or various degrees of saturation were without effect. Specific inhibitors of cyclooxygenase-, lipoxygenase-, and cytochrome P450-dependent metabolism did not affect arachidonic acid-induced JNK activity. We further demonstrated that the free radical scavenger N-acetylcysteine blocked arachidonic acid-induced JNK activation, while H2O2, a reactive oxidative molecule, activated JNK in a dose-dependent manner, providing additional support for a redox mechanism. Moreover, arachidonic acid activated NADPH oxidase (EC 1.6.-.-, EC 1.6.99.-) in a dose-dependent manner, and the potency of superoxide generation paralleled that of JNK activation by other fatty acids. We conclude that in kidney epithelial cells arachidonic acid activates JNK by means of NADPH oxidase and superoxide generation, independent of eicosanoid biosynthesis.
Keywords: fatty acids, phospholipase A2, G protein-coupled receptors, superoxide anion
Arachidonic acid, an important lipid messenger, is generated in response to a variety of stimuli, including vasoactive agonists linked to G protein-coupled receptors, inflammatory agents, and cytokines (1–3). Arachidonic acid modulates growth (4, 5), and recent evidence suggests the involvement of the superfamily of mitogen-activated protein kinases (MAPKs) in arachidonic acid-induced growth, since p42MAPK and p44MAPK (ERK) were reported to be activated following stimulation (6, 7). This cascade is currently considered to involve the activation of ras, raf-1, and MEK, leading to MAPK (8). The upstream pathway linked to arachidonic acid has not been precisely defined in kidney epithelium, but appears to require Ca2+ mobilization (N. Dulin and J.G.D., unpublished data). A second subgroup of the MAPK superfamily, c-jun N-terminal kinase (JNK), is activated by oncoproteins, stress (e.g., ultraviolet exposure, osmolarity burden, heat shock, and radiation) and proinflammatory cytokines (e.g., tumor necrosis factor and interleukin 1) (9–14). Following activation, JNK phosphorylates transcription factors c-jun, elk-1, and activating factor 2 (ATF-2) (15–22). JNK modulates T cell function (23), hepatic regeneration (24), mitogenesis linked to G protein-coupled receptors (25–28), and programmed cell death—i.e., apoptosis (18, 29–31). It was recently demonstrated that JNK activation involves the rho subgroup of ras-like GTPases, including rac and cdc42 (32–35). In phagocytes arachidonic acid translocates rac from cytosol to membrane and activates NADPH oxidase (EC 1.6.-.-, EC 1.6.99-), thus, making arachidonic acid a potential regulator of the JNK pathway (36–40).
In rabbit kidney proximal tubular epithelial cells, arachidonic acid is released rapidly by angiotensin II after activation of a membrane-associated phospholipase A2 (PLA2) (refs. 41 and 42 and S. Harwalkar, and J.G.D., unpublished data). Because of the importance of arachidonic acid in epithelial cell signaling, we evaluated the potential for its involvement in JNK activation by assessing the phosphorylation of the transcription factor ATF-2. We demonstrated that arachidonic acid and linoleic acid activate JNK specifically, while other fatty acids from n − 3 and n − 6 series and/or various degrees of saturation were ineffective. The mechanism appears to involve superoxide generation after activation of NADPH oxidase, and it is independent of downstream eicosanoid biosynthesis.
METHODS
Materials.
Arachidonic acid (tissue culture grade), N-acetylcysteine, purified rabbit IgG, anti-rabbit IgG agarose beads, and luciginin were purchased from Sigma. Collagenase I, soybean trypsin inhibitor, cell culture medium, and additives were from GIBCO/BRL. Bovine serum albumin (fatty acid-free) was purchased from Boehringer Mannheim. Linoleic acid, eicosa-(11Z,14Z)-dienoic acid, eicosa-(11Z,14Z,17Z)-trienoic acid, stearic acid, indomethacin, cinnamyl-3,4-dihydroxy-α-cyanocinnamate, and ketoconazole were obtained from Biomol (Plymouth Meeting, PA). [γ-32P]ATP was from DuPont/NEN. Rabbit anti-JNK1(FL) polyclonal antibody, which cross-reacts with all three isoforms of JNK, and recombinant ATF-2 were purchased from Santa Cruz Biotechnology.
Cell Culture.
Primary culture of rabbit proximal tubular epithelial cells were prepared as previous described (43). They were maintained in modified DMEM/F12 (1:1) media supplemented with 5% fetal calf serum, 5 μg/ml insulin, 5 μg/ml transferrin, 0.5 μM hydrocortisone, 350 μg/ml l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were passaged once into 100-mm Petri dishes and were employed as subconfluent monolayers.
Measurement of Superoxide Anion Production in Intact Cells.
O2̇− production was determined according to a published method with some modifications (44). Cells were washed twice with Hanks balanced salt solution containing no calcium and magnesium. They were harvested by incubating with 2 ml of the same buffer containing 2 mg/ml collagenase I, 2 mg/ml soybean trypsin inhibitor, and 4 mg/ml BSA and were pelleted by centrifugation at 200 × g for 5 min at 4°C. After the pellet had been resuspended in assay buffer containing 130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.5 mM CaCl2, 35 mM phosphoric acid, and 20 mM Hepes (pH 7.4), the cells were counted and centrifuged again at 200 × g for 5 min at 4°C. They were resuspended in the same buffer and stored on ice until use. The measurement of superoxide generation was carried out in the darkroom with a Lumat LB 9501 luminometer. To start the assay, dark-adapted luciginin (final concentration 250 μM) was added to 1 × 106 cells followed by fatty acid (vehicle: ethanol) in a total volume of 1 ml of assay buffer. Photo emission in terms of relative light units (RLU) was measured every minute for 10 min. The standard curve for superoxide generation was checked with the xanthine/xanthine oxidase system as described previously (45).
NADPH Oxidase Assay.
Cells were washed twice in ice-cold Dulbecco’s phosphate-buffered saline and were scraped from the plate in the same solution followed by centrifugation at 750 × g, 4°C, for 10 min. The pellet was resuspended in buffer containing 20 mM KH2PO4, 1 mM EGTA, 10 μg/ml aprotinin, 25 μg/ml leupeptin, and 1 mM phenylmethanesulfonyl fluoride. The cell suspension was homogenized with 50 strokes in a Dounce homogenizer on ice, and aliquots of the homogenate were used immediately. To start the assay, 100 μl of homogenate was added into 900 μl of 50 mM phosphate buffer, pH 7.0, containing 1 mM EGTA, 150 mM sucrose, 500 μM luciginin, and 100 μM NADPH, in the presence or absence of arachidonic acid or superoxide dismutase (SOD; 50 units) (44). Photoemission in terms of RLU was measured every minute for 15 min. There was a slight increase of photoemission in controls without fatty acids with NADPH as substrate. This effect was not the result of the presence of trace amount of ethanol because the same increase was seen in the absence of ethanol; it may due to the presence of activators in the cell lysate when NADPH was available. Protein content was normalized by BCA assay (Pierce) according to manufacturer’s instructions.
Immunoprecipitation and Immune Complex Kinase Assays of JNK.
Rabbit proximal tubular epithelial cells were serum starved in media prior to treatment. Preliminary studies showed JNK activation induced by arachidonic acid was similar when cells were serum starved for 2 to 24 hr. The kinase assay was carried out according to a previously published method with slight modifications (20, 21). In brief, following treatments indicated in figure legends, cells were washed twice with ice-cold Dulbecco’s phosphate-buffered saline and lysed by adding 0.3 ml of buffer containing 50 mM Tris·HCl (pH 7.2), 1% (wt/wt) Triton X-100, 1 mM Na3VO4, 1 mM EGTA, 0.2 mM phenylmethanesulfonyl fluoride, 25 μg/ml leupeptin, and 10 μg/ml aprotinin. Protein contents were determined by BCA assay. Cell lysates containing 200 μg of protein in a total volume of 800 μl were precleared with 1 μg of nonimmune rabbit IgG and 30 μl of goat anti-rabbit IgG agarose beads on a rotating plate for 1.5 hr at 4°C. After centrifugation at 10,000 × g for 10 min, the supernatant was mixed with 1 μg of anti-JNK1(FL) polyclonal antibody and 25 μl of goat anti-rabbit IgG agarose beads on a rotating plate overnight at 4°C. The beads should bind all three isoforms of JNK, since the antibody is cross-reactive. They were pelleted by centrifugation at 10,000 × g for 5 min and were washed two times with the lysis buffer, one time with the kinase assay buffer containing 20 mM Hepes (pH 7.6), 20 mM MgCl2, 25 mM β-glycerol phosphate, 0.1 mM Na3VO4, and 2 mM dithiothreitol. The kinase assay was carried out at 30°C for 15 min in 30 μl of assay buffer containing 0.5 μg of ATF-2, 20 μM ATP, and 2 μCi (1 μCi = 37 kBq) of [γ-32P]ATP. The reaction was terminated by addition of Laemmli’s sample buffer followed by boiling for 5 min. The samples were resolved by SDS/10% polyacrylamide gel electrophoresis (SDS-PAGE), stained with Coomassie brilliant blue, and exhaustively destained. The gel was dried and the incorporation of 32P was visualized by autoradiography. Gel slices of the 69-kDa ATF-2 bands were also cut out in most of the experiments, and the radioactivity was measured by liquid scintillation counting.
Statistics.
32P incorporation into the substrate ATF-2 was analyzed as paired data between control and fatty acid-stimulated groups by the Wilcoxon signed rank test. Significance was determined as probability (P) less than 0.05.
RESULTS
Effects of Arachidonic Acid and Other Fatty Acids on JNK Activation.
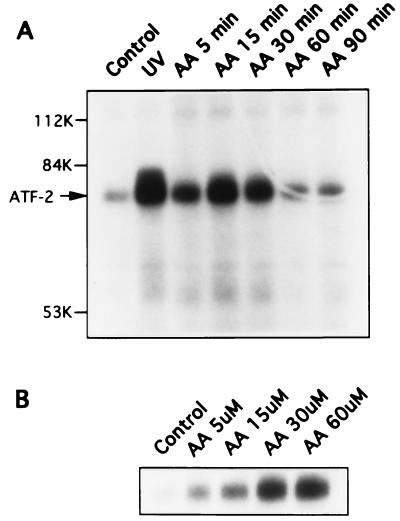
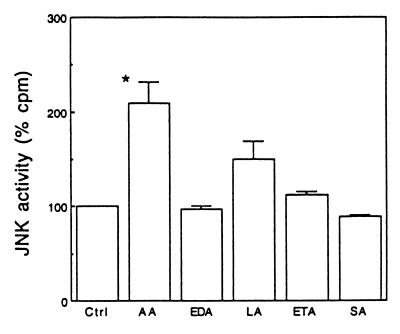
As shown in Fig. 1A, exposure of cells to 30 μM arachidonic acid activated JNK in a time-dependent manner as assayed by monitoring 32P incorporation into ATF-2. JNK activation was evident at 5 min (155% of control, n = 2) and peaked at 15 min (209% of control, n = 8). At 60 min, JNK activity returned to the basal level. JNK activation by arachidonic acid was also dose-dependent in that an increment in ATF-2 phosphorylation was observed at 5 μM arachidonic acid and maximal at 30 μM (Fig. 1B). It has been reported that polyunsaturated fatty acids of the n − 3 and n − 6 series, in addition to arachidonic acid, activate signaling, including calcium mobilization, protein kinase C, and ERK (6). To determine whether JNK activation is unique to arachidonic acid, we compared various fatty acids at 30 μM and observed that arachidonic acid was the most potent stimulator of JNK activity (209% of control, n = 8, P < 0.05) (Fig. 2). Linoleic acid, a precursor of arachidonic acid, also activated JNK, but was less potent (150% of control, n = 4). Eicosa-(11Z,14IZ)-dienoic acid (n = 4), eicosa-(11Z,14Z,17Z)-trienoic acid (n = 3), and stearic acid (n = 3), a saturated fatty acid, had no significant effect. These observations demonstrate that JNK activation is specific to arachidonic acid and linoleic acid.
Figure 1.
Time (A) and dose (B) -dependent activation of JNK by arachidonic acid. The figure represents two similar experiments. (A) Cells were exposed to 30 μM arachidonic acid (AA) for the time indicated on the top of the gel or 160 J/cm2 ultraviolet (UV) for 30 minutes. Cell lysates were immunoprecipitated with anti-JNK1 polyclonal antibody and the in vitro kinase assay was carried out. Equal protein loading was confirmed by staining the SDS/10% polyacrylamide gel with Coomassie brilliant blue before drying and exposure to Kodak X-Omat AR film. The 32P-phosphorylated substrate ATF-2 is indicated on the autoradiograph with an arrow. (B) Cells were treated with various concentrations of arachidonic acid (5–60 μM) for 15 min and assayed for JNK activity as described above.
Figure 2.
Fatty acid specificity. Cells were exposed for 15 min to 30 μM arachidonic acid (AA), eicosa-(11Z,14Z)-dienoic acid (EDA), linoleic acid (LA), eicosa-(11Z,14Z,17Z)-trienoic acid (ETA), and stearic acid (SA), respectively. Data show the percentage of 32P incorporation. ∗, P < 0.05.
Arachidonic Acid Metabolism Is Not Necessary for JNK activation.
In most mammalian cells, arachidonic acid is oxidized through the cyclooxygenase, lipoxygenase, and/or cytochrome P450 pathways to yield eicosanoids that mediate biological effects. In rabbit proximal tubular epithelial cells the predominant pathway of arachidonic acid metabolism is through the cytochrome P450 pathway, while cyclooxygenase and lipoxygenase activities are minimal (46). To evaluate the contributions of these metabolic pathways, cells were treated with the cyclooxygenase inhibitor indomethacin, the lipoxygenase inhibitor cinnamyl-3,4-dihydroxy-α-cyanocinnamate, or the cytochrome P450 inhibitor ketoconazole for 30 min, followed by stimulation with 30 μM arachidonic acid. None of the inhibitors significantly influenced arachidonic acid-induced JNK activity, at doses known to abrogate eicosanoid biosynthesis in mammalian cells, including epithelial cells (47–49) (Table 1). Ketoconazole (100 μM) was ineffective at blocking JNK, despite the fact that p42 and p44 MAPK activation (N. Dulin and J.G.D., unpublished data) as well as calcium mobilization were abolished with this concentration (43). Thus, we concluded that arachidonic acid-induced JNK activation did not require subsequent eicosanoid biosynthesis.
Table 1.
Effects of metabolic pathway inhibitors on arachidonic acid-induced JNK activation
| Inhibitor* | JNK activity, % of AA† | n |
|---|---|---|
| Cyclooxygenase inhibitor | ||
| Indomethacin (20 μM) | 101 ± 19 | 3 |
| Lipoxygenase inhibitor | ||
| CDC (16 μM) | 98–126 | 2 |
| Cytochrome P450 inhibitor | ||
| Ketoconazole (20 μM) | 118–149 | 2 |
Quiescent rabbit tubular epithelial cells were preincubated with inhibitors for 30 min before exposure to 30 μM arachidonic acid. CDC, cinnamyl-3,4-dihydroxy-α-cyanocinnamate.
Results are shown as mean ± SD where n = 3 or as range where n = 2 of the percentage of 32P incorporation, with arachidonic acid (AA) as 100%.
Arachidonic Acid Activates JNK Through a Redox-Related Mechanism.
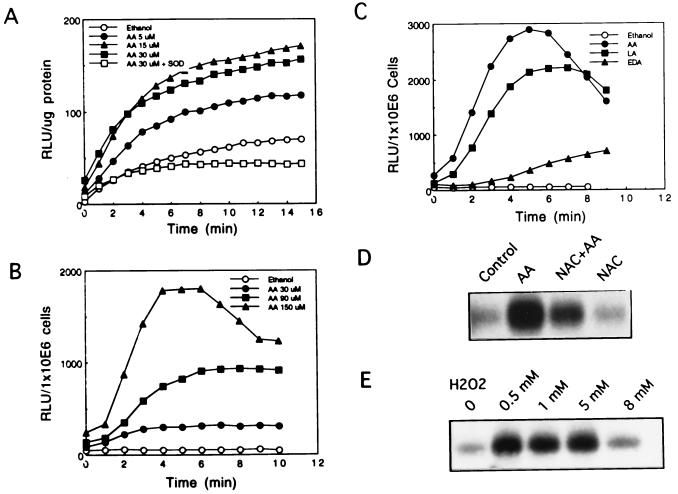
Protein kinases, especially protein kinase C (PKC), have been implicated in arachidonic acid-induced signaling in mammalian cells (50). Therefore, we assessed the effect of PKC inhibitors on arachidonic acid-induced JNK activation. Neither staurosporine at 0.15 μM (51), a concentration known to inhibit angiotensin II- and phorbol 12-tetradecanoate 13-acetate-induced MAPK (ERK1, ERK2) activation in these cells, nor other PKC inhibitors such as GF10923X (0.5 μM), H7 (75 μM), and HA1004 (75 μM) blocked arachidonic acid’s effect on JNK (data not shown). Since it has been demonstrated that arachidonic acid and angiotensin II activate membrane-associated NADPH oxidase to generate superoxide anion, independent of PKC, in phagocytes (36, 40) and smooth muscle cells (44), we assessed whether arachidonic acid activation of JNK was mediated by NADPH oxidase. Employing a crude cell lysate, we demonstrated maximal superoxide anion generation at 7 min with 15 μM arachidonic acid. Thirty micromolar or higher concentrations were less effective (Fig. 3A and data not shown). In addition, superoxide dismutase abrogated arachidonic acid-induced photoemission, thereby confirming its identity. We also observed that arachidonic acid induced rapid dose-dependent superoxide generation in intact cells, which peaked at 6 min. A 6- to 9-fold increase of superoxide generation by 30 μM arachidonic acid and 36-fold increase by up to 150 μM arachidonic acid were observed (Fig. 3B). Most importantly, the potency of superoxide anion generation paralleled activation of JNK by fatty acids. Arachidonic acid was the most potent, followed by linoleic acid, while other fatty acids were of negligible potency (Fig. 3C and data not shown). In addition, we observed that arachidonic acid’s effect on JNK activation was blocked by the antioxidant N-acetylcysteine. As N-acetylcysteine is an acidic compound, we had applied it in two ways. Without adjusting pH of the medium (as probably most researchers do), we consistently observed 100% inhibition, while basal JNK activity was not influenced by N-acetylcysteine alone. When the medium pH was adjusted to neutral, N-acetylcysteine blocked at least 66% of arachidonic acid-induced JNK activation (Fig. 3D). Despite the fact that 20 mM N-acetylcysteine is the most common dosage used, we found that 10 mM was partially effective (up to 74% inhibition). Moreover, H2O2 activated JNK in a dose-dependent manner (Fig. 3E), providing additional support for the involvement of a redox mechanism of activation.
Figure 3.
Involvement of oxidative stress. (A) NADPH oxidase activity. Crude cell lysates were incubated with luciginin (500 μM) and NADPH (100 μM) in the presence or absence of arachidonic acid (5–30 μM) or superoxide dismutase (SOD, 50 units). Superoxide generation was determined by photoemission (RLU) every 1 min. (B) Arachidonic acid-induced superoxide generation in intact cells. The cells (1 × 106) were incubated with various concentrations of arachidonic acid. Superoxide generation was determined by photoemission (RLU) every 1 min. (C) Specificity of fatty acids in superoxide generation. Cells (1 × 106) were incubated with 240 μM arachidonic acid (AA), linoleic acid (LA), or eicosa-(11Z,14Z)-dienoic acid (EDA). Superoxide generation was determined by photoemission (RLU) every 1 min. (D) Effects of antioxidant on arachidonic acid-induced JNK activation. Cells were pretreated with 20 mM N-acetylcysteine (NAC) for 30 min followed by 30 μM arachidonic acid for 15 min. Cell lysates were immunoprecipitated with anti-JNK1 polyclonal antibody and the in vitro kinase assay was carried out. (E) Effects of hydrogen peroxide on JNK activation. Cells were exposed to 0.5 mM, 1 mM, 5 mM, or 8 mM hydrogen peroxide (H2O2) for 10 min. Cell lysates were immunoprecipitated with anti-JNK1 polyclonal antibody and the in vitro kinase assay was carried out. Every panel of the figure is a representative of at least two similar experiments.
DISCUSSION
These observations demonstrate that arachidonic acid and linoleic acid activate JNK independent of eicosanoid metabolism. The underlying mechanism appears to involve superoxide anion generation following activation of NADPH oxidase. Support for this hypothesis is provided by the following observations: (i) the pattern of superoxide generation paralleled the pattern of JNK activation by various fatty acids; (ii) N-acetylcysteine, an antioxidant, blocked arachidonic acid-induced JNK activation; and (iii) H2O2 mimicked the ability of arachidonic acid to activate JNK. There is a general agreement that oxidative stress influences mammalian cell proliferation. Early response genes, such as c-jun and c-fos, are induced by exposure of cells to oxidative agents through AP-1 and/or NF-κB activation (52–55). Recent studies support p21ras as a common signaling target of reactive free radicals (56). However, the involvement of free radicals in JNK induction appears to be quite cell-specific, since H2O2 is either ineffective in NIH 3T3 cells (57) or inhibitory to heat shock-induced JNK activity in 3T3–4A cells (12). Our data provide a cellular mechanism for the recent observations of Pombo et al. (58) demonstrating renal JNK activation by oxidative stress after ischemia and reperfusion.
NADPH oxidase has been intensively studied in phagocytes, while little is known about its role in other cell types, including kidney epithelial cells. Activation of this enzyme requires the coordinative assembly of four structural proteins: a membrane-bound flavocytochrome b558 and three cytosolic proteins p47phox, p67phox, and rac, a GTP-binding regulatory protein (59). Of these proteins, rac is the only one that has been implicated independently in JNK activation (32–35) and in arachidonic acid signaling (5, 27). During basal conditions, rac is maintained in an inactive form associated with its inhibitory protein Rho-GDI. After activation, the complex dissociates and rac translocates to the plasma membrane, serving as one of the components in NADPH oxidase activation (38). On the other hand, arachidonic acid has been shown to be the immediate activator of the membrane NADPH oxidase in phagocytes (36, 40). In a cell-free system arachidonic acid translocated rac from the cytosol to the membrane, and this was potentiated by γ-thio-GTP (39). However, the sequence that links arachidonic acid, rac, NADPH oxidase, and the upstream G protein-coupled receptors needs to be further defined. For example, Bos and co-workers (5) concluded that epidermal growth factor (EGF)-induced arachidonic acid release and leukotriene production were mediated by rac in fibroblasts, since a dominant-negative mutant of rac blocked EGF’s effects. Employing a crude cell lysate, we observed the arachidonic acid-induced activation of NADPH oxidase. The maximum effect was reached at 15 μM arachidonic acid, lower than that noticed with intact cells. This difference in potency may relate to better access of arachidonic acid to critical enzymes in homogenates in contrast to intact cells. Our data strongly suggest that arachidonic acid activates JNK through NADPH oxidase. However, the importance of rac in NADPH oxidase activation and other aspects of signaling in epithelial cells remains unknown at this point.
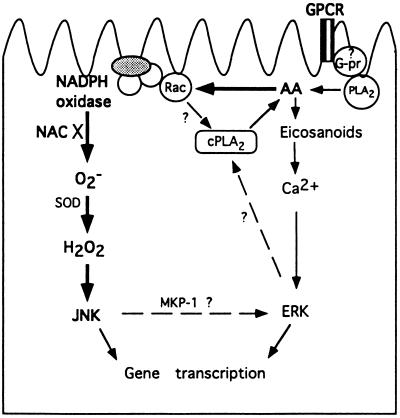
Previous work from our laboratory demonstrated that angiotensin II releases arachidonic acid through activating a membrane-associated PLA2, leading to the activation of the ERK group of MAPK in proximal tubular epithelium. Arachidonic acid-induced ERK activation is rapid, calcium dependent, and PKC independent, and requires further metabolism of arachidonic acid through the lipoxygenase and the cytochrome P450 pathways (N. Dulin and J.G.D., unpublished data). An alternative model has been proposed in CHO cells, whereby cytosolic PLA2 is phosphorylated and activated after receptor-mediated signaling and phosphorylation of ERK (60) (see cellular model, Fig. 4). JNK has also been observed to be activated by G protein-coupled receptors (24–26, 61). However, we demonstrated that arachidonic acid-induced JNK activation is slower than ERK phosphorylation, and is independent of both PKC and eicosanoids. Our unpublished data also indicate a calcium-independent mechanism. These observations support the hypothesis that JNKs and ERKs are regulated independently in kidney epithelial cells. In fibroblasts, Bokemeyer et al. (62) provided a mechanism of interaction through induction of MKP-1, a phosphatase that specifically dephosphorylates ERK, by JNK. Thus, we propose a signal pathway wherein arachidonic acid has independent effects on ERK and JNK after activation of a G protein-coupled receptors. However, further investigation is warranted to gain a better understanding of the detailed interactions of these two kinase cascades in epithelial cells.
Figure 4.
Schematic model of arachidonic acid (AA)-mediated MAPK activation in rabbit proximal tubule epithelial cells. The elements linked by bold arrows were employed in our experiments, while the rest were indicated by work done in this laboratory and described in the literature. NAC, N-acetyl cysteine; SOD, superoxide dismutase; cPLA2, cytosolic PLA2; GPCR, G protein-coupled receptor; G-pr, G protein.
In summary, we demonstrate that arachidonic acid induces JNK activation in rabbit proximal tubular epithelial cells through a redox mechanism rather than through classical eicosanoid biosynthesis. We conclude that NADPH oxidase is responsible, at least in part, for arachidonic acid-induced superoxide generation and JNK activation.
Acknowledgments
We thank Judy Preston for expert assistance with cell isolation and tissue culture and Pearl M. Whitley for technical assistance. We also thank co-workers Drs. Nickolai Dulin and Subash Harwalkar for sharing their unpublished information. This work was supported by National Institutes of Health Grant HL41618 and Training Grant HL07714.
ABBREVIATIONS
- PLA2
phospholipase A2
- JNK
c-jun N-terminal kinase or stress-activated protein kinase (SAPK)
- MAPK
mitogen-activated protein kinase, a superfamily that includes p42MAPK and p44MAPK (also known as ERK), JNK/SAPK, and p38/HOG SAPKs
- ATF-2
activating factor 2
- RLU
relative light unit(s)
- PKC
protein kinase C
References
- 1.Piomelli D. Curr Opin Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay G K, Hwang S I, Imagawa W, Nandi S. Prostaglandins Leukotrienes Essent Fatty Acids. 1993;48:71–78. doi: 10.1016/0952-3278(93)90012-l. [DOI] [PubMed] [Google Scholar]
- 3.Graber C S R, Nunez E. Prostaglandins Leukotrienes Essent Fatty Acids. 1993;48:117–122. doi: 10.1016/0952-3278(93)90019-s. [DOI] [PubMed] [Google Scholar]
- 4.Graber M N, Alfonso A, Gill D L. J Biol Chem. 1996;271:883–888. doi: 10.1074/jbc.271.2.883. [DOI] [PubMed] [Google Scholar]
- 5.Peppelenbosch M P, Qiu R-G, de Vries-Smits A M M, Tertoolen L G J, de Laat S W, McCormick F, Hall A, Symons M H, Bos J L. Cell. 1995;81:849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 6.Hii C S, Ferrante A, Edwards Y S, Huang Z H, Hartfield P J, Rathjen D A, Poulos A, Murray A W. J Biol Chem. 1995;270:4201–4204. doi: 10.1074/jbc.270.9.4201. [DOI] [PubMed] [Google Scholar]
- 7.Rao G N, Baas A S, Glasgow W C, Eling T E, Runge M S, Alexander R W. J Biol Chem. 1994;269:32586–32591. [PubMed] [Google Scholar]
- 8.Avruch J, Zhang X F, Kyriakis J M. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 10.Galcheva Gargova Z, Derijard B, Wu I H, Davis R J. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 11.Kharbanda S, Saleem A, Shafman T, Emoto Y, Taneja N, Rubin E, Weichselbaum R, Woodgett J, Avruch J, Kyriakis J, Kufe D. J Biol Chem. 1995;270:18871–18874. doi: 10.1074/jbc.270.32.18871. [DOI] [PubMed] [Google Scholar]
- 12.Adler V, Schaffer A, Kim J, Dolan L, Ronai Z. J Biol Chem. 1995;270:26071–26077. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- 13.Sluss H K, Barrett T, Derijard B, Davis R J. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westwick J K, Weitzel C, Minden A, Karin M, Brenner D A. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 15.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 16.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Nature (London) 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 17.Cano E, Hazzalin C A, Kardalinou E, Buckle R S, Mahadevan L C. J Cell Sci. 1995;108:3599–3609. doi: 10.1242/jcs.108.11.3599. [DOI] [PubMed] [Google Scholar]
- 18.Adler V, Fuchs S Y, Kim J, Kraft A, King M P, Pelling J, Ronai Z. Cell Growth Differ. 1995;6:1437–1446. [PubMed] [Google Scholar]
- 19.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Campbell D, Derijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 21.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livingstone C, Patel G, Jones N. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben Neriah Y. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 24.Westwick J K, Weitzel C, Leffert H L, Brenner D A. J Clin Invest. 1995;95:803–810. doi: 10.1172/JCI117730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coso O A, Chiariello M, Kalinec G, Kyriakis J M, Woodgett J, Gutkind J S. J Biol Chem. 1995;270:5620–5624. doi: 10.1074/jbc.270.10.5620. [DOI] [PubMed] [Google Scholar]
- 26.Bogoyevitch M A, Ketterman A J, Sugden P H. J Biol Chem. 1995;270:29710–29717. doi: 10.1074/jbc.270.50.29710. [DOI] [PubMed] [Google Scholar]
- 27.Nishio E, Nakata H, Arimura S, Watanabe Y. Biochem Biophys Res Commun. 1996;219:277–282. doi: 10.1006/bbrc.1996.0223. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell F M, Russell M, Johnson G L. Biochem J. 1995;309:381–384. doi: 10.1042/bj3090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Meyer C F, Tan T. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 31.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 32.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 33.Minden A, Lin A, Claret F X, Abo A, Karin M. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 34.Bagrodia S, Derijard B, Davis R J, Cerione R A. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 35.Olson M F, Ashworth A, Hall A. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 36.Henderson L M, Moule S K, Chappell J B. Eur J Biochem. 1993;211:157–162. doi: 10.1111/j.1432-1033.1993.tb19882.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubinek T, Levy R. Biochim Biophys Acta. 1993;1176:51–58. doi: 10.1016/0167-4889(93)90176-p. [DOI] [PubMed] [Google Scholar]
- 38.Abo A, Webb M R, Grogan A, Segal A W. Biochem J. 1994;298:585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawai T, Asada M, Nunoi H, Matsuda I, Ando S, Sasaki T, Kaibuchi K, Takai Y, Katayama K. Biochem Biophys Res Commun. 1993;195:264–269. doi: 10.1006/bbrc.1993.2039. [DOI] [PubMed] [Google Scholar]
- 40.Sakata A, Ida E, Tominaga M, Onoue K. J Immunol. 1987;138:4353–4359. [PubMed] [Google Scholar]
- 41.Jacobs L S, Douglas J G. Hypertension. 1996;28:663–668. doi: 10.1161/01.hyp.28.4.663. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Wang Y-P, Capparelli A W, Jo O D, Yanagawa N. Am J Physiol. 1994;266:F202–F209. doi: 10.1152/ajprenal.1994.266.2.F202. [DOI] [PubMed] [Google Scholar]
- 43.Madhun Z T, Goldthwait D A, McKay D, Hopfer U, Douglas J G. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griendling K K, Minieri C A, Ollerenshaw J D, Alexander R W. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 45.Ohara Y, Peterson T E, Harrison D G. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero M, Hopfer U, Madhun Z, Zhou W, Douglas J G. Renal Physiol Biochem. 1996;14:199–207. doi: 10.1159/000173405. [DOI] [PubMed] [Google Scholar]
- 47.Civan M M, Coca-Prados M, Peterson-Yantorno K. Invest Ophthalmol Vis Sci. 1994;35:2876–2886. [PubMed] [Google Scholar]
- 48.Hata Y, Ota S, Nagata T, Uehare Y, Terano A, Sugimoto T. Prostaglandins. 1993;45:129–141. doi: 10.1016/0090-6980(93)90028-6. [DOI] [PubMed] [Google Scholar]
- 49.Capdevilla J L, Gill L, Orellana M, Marnett L J, Mason J I, Yadagiri P, Faly J R. Arch Biochem Biophys. 1996;261:257–263. doi: 10.1016/0003-9861(88)90340-2. [DOI] [PubMed] [Google Scholar]
- 50.Seifert R, Schaechtele C, Rosenthal W, Schultz G. Biochem Biophys Res Commun. 1988;154:20–26. doi: 10.1016/0006-291x(88)90643-2. [DOI] [PubMed] [Google Scholar]
- 51.Force T, Kyriakis J M, Avruch J, Bonventre J V. J Biol Chem. 1991;266:6650–6656. [PubMed] [Google Scholar]
- 52.Burdon R H. Free Radical Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 53.Goldstone S D, Fragonas J C, Jeitner T M, Hunt N H. Biochim Biophys Acta. 1995;1263:114–122. doi: 10.1016/0167-4781(95)00088-x. [DOI] [PubMed] [Google Scholar]
- 54.Rao G N, Lassegue B, Griendling K K, Alexander R W. Oncogene. 1993;8:2759–2764. [PubMed] [Google Scholar]
- 55.Rao G N, Lassegue B, Griendling K K, Alexander R W, Berk B C. Nucleic Acids Res. 1993;21:1259–1263. doi: 10.1093/nar/21.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lander H M, Ogiste J S, Teng K K, Novogrodsky A. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 57.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 58.Pombo C M, Bonventre J V, Avruch J, Woodgett J R, Kyriakis J M, Force T. J Biol Chem. 1994;269:26546–26551. [PubMed] [Google Scholar]
- 59.Quinn M T, Evans T, Loetterle L R, Jesaitis A J, Bokoch G M. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- 60.Lin L, Wartmann M, Lin A, Knopf J L, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 61.Zohn I E, Yu H, Li X, Cox A D, Earp H S. Mol Cell Biol. 1995;15:6160–6168. doi: 10.1128/mcb.15.11.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bokemeyer D, Sorokin A, Yan M, Ahn N G, Templeton D J, Dunn M J. J Biol Chem. 1996;271:639–642. doi: 10.1074/jbc.271.2.639. [DOI] [PubMed] [Google Scholar]