Abstract
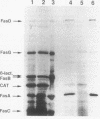
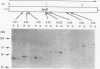
The FasD protein is essential for the biogenesis of 987P fimbriae of Escherichia coli. In this study, subcellular fractionation was used to demonstrate that FasD is an outer membrane protein. In addition, the accessibility of FasD to proteases established the presence of surface-exposed FasD domains on both sides of the outer membrane. The fasD gene was sequenced, and the deduced amino acid sequence was shown to share homologous domains with a family of outer membrane proteins from various fimbrial systems. Similar to porins, fimbrial outer membrane proteins are relatively polar, lack typical hydrophobic membrane-spanning domains, and posses secondary structures predicted to be rich in turns and amphipathic beta-sheets. On the basis of the experimental data and structural predictions, FasD is postulated to consist essentially of surface-exposed turns and loops and membrane-spanning interacting amphipathic beta-strands. In an attempt to test this prediction, the fasD gene was submitted to random in-frame linker insertion mutagenesis. Preliminary experiments demonstrated that it was possible to produce fasD mutants, whose products remain functional for fimbrial export and assembly. Subsequently, 11 fasD alleles, containing linker inserts encoding beta-turn-inducing residues, were shown to express functional proteins. The insertion sites were designated permissive sites. The inserts used are expected to be least detrimental to the function of FasD when they are inserted into surface-exposed domains not directly involved in fimbrial export. In contrast, FasD is not expected to accommodate such residues in its amphipathic beta-strands without being destabilized in the membrane and losing function. All permissive sites were sequenced and shown to be located in or one residue away from predicted turns. In contrast, 5 of 10 sequenced nonpermissive sites were mapped to predicted amphipathic beta-strands. These results are consistent with the structural predictions for FasD.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Charbit A., Ronco J., Michel V., Werts C., Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991 Jan;173(1):262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Reid G. K., Campana A. Export of hybrid proteins FhuA'-'LacZ and FhuA'-'PhoA to the cell envelope of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2267–2275. doi: 10.1128/jb.170.5.2267-2275.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Dodd D. C., Bassford P. J., Jr, Eisenstein B. I. Dependence of secretion and assembly of type 1 fimbrial subunits of Escherichia coli on normal protein export. J Bacteriol. 1984 Sep;159(3):1077–1079. doi: 10.1128/jb.159.3.1077-1079.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson K. W., Jacob-Dubuisson F., Striker R. T., Hultgren S. J. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Makowski L. Helical structure of P pili from Escherichia coli. Evidence from X-ray fiber diffraction and scanning transmission electron microscopy. J Mol Biol. 1992 Dec 5;228(3):735–742. doi: 10.1016/0022-2836(92)90860-m. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Kuehn M. J., Brändén C. I., Hultgren S. J. Conserved immunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J. 1992 Apr;11(4):1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Normark S., Abraham S. N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- Jalajakumari M. B., Thomas C. J., Halter R., Manning P. A. Genes for biosynthesis and assembly of CS3 pili of CFA/II enterotoxigenic Escherichia coli: novel regulation of pilus production by bypassing an amber codon. Mol Microbiol. 1989 Dec;3(12):1685–1695. doi: 10.1111/j.1365-2958.1989.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Jacob-Dubuisson F., Dodson K., Kuehn M., Slonim L., Striker R., Hultgren S. J. Adhesin presentation in bacteria requires molecular chaperones and ushers. Infect Immun. 1992 Nov;60(11):4445–4451. doi: 10.1128/iai.60.11.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990 Dec;4(12):2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Karlyshev A. V., Galyov E. E., Smirnov OYu, Guzayev A. P., Abramov V. M., Zav'yalov V. P. A new gene of the f1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 1992 Feb 3;297(1-2):77–80. doi: 10.1016/0014-5793(92)80331-a. [DOI] [PubMed] [Google Scholar]
- Kaufman M. R., Seyer J. M., Taylor R. K. Processing of TCP pilin by TcpJ typifies a common step intrinsic to a newly recognized pathway of extracellular protein secretion by gram-negative bacteria. Genes Dev. 1991 Oct;5(10):1834–1846. doi: 10.1101/gad.5.10.1834. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Koebnik R., Braun V. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1993 Feb;175(3):826–839. doi: 10.1128/jb.175.3.826-839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M. G., Pugsley A. P. The normally periplasmic enzyme beta-lactamase is specifically and efficiently translocated through the Escherichia coli outer membrane when it is fused to the cell-surface enzyme pullulanase. Mol Microbiol. 1990 Jul;4(7):1101–1109. doi: 10.1111/j.1365-2958.1990.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Normark S., Hultgren S. J. Immunoglobulin-like PapD chaperone caps and uncaps interactive surfaces of nascently translocated pilus subunits. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wijfjes A., de Graaf F. K. Identification and characterization of precursors in the biosynthesis of the K88ab fimbria of Escherichia coli. J Bacteriol. 1983 Apr;154(1):41–49. doi: 10.1128/jb.154.1.41-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. K., Klebba P. E. Export of FepA::PhoA fusion proteins to the outer membrane of Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):5894–5900. doi: 10.1128/jb.171.11.5894-5900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K., Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11(2):95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992 Feb;6(4):435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Adhesins as lectins: specificity and role in infection. Curr Top Microbiol Immunol. 1990;151:91–113. doi: 10.1007/978-3-642-74703-8_5. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Parsot C., Taxman E., Mekalanos J. J. ToxR regulates the production of lipoproteins and the expression of serum resistance in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1641–1645. doi: 10.1073/pnas.88.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux C. R., Friedrich M. J., Kadner R. J. Genes on the 90-kilobase plasmid of Salmonella typhimurium confer low-affinity cobalamin transport: relationship to fimbria biosynthesis genes. J Bacteriol. 1990 Nov;172(11):6217–6222. doi: 10.1128/jb.172.11.6217-6222.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal B., de Graaf F. K. The nucleotide sequence of the fanD gene encoding the large outer membrane protein involved in the biosynthesis of K99 fimbriae. Nucleic Acids Res. 1989 Feb 11;17(3):1263–1263. doi: 10.1093/nar/17.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Structural and functional properties of porin channels in E. coli outer membranes. Experientia. 1990 Feb 15;46(2):167–173. [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schifferli D. M., Abraham S. N., Beachey E. H. Use of monoclonal antibodies to probe subunit- and polymer-specific epitopes of 987P fimbriae of Escherichia coli. Infect Immun. 1987 Apr;55(4):923–930. doi: 10.1128/iai.55.4.923-930.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H., Taylor R. K. 987P fimbrial gene identification and protein characterization by T7 RNA polymerase-induced transcription and TnphoA mutagenesis. Mol Microbiol. 1991 Jan;5(1):61–70. doi: 10.1111/j.1365-2958.1991.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H., Taylor R. K. Genetic analysis of 987P adhesion and fimbriation of Escherichia coli: the fas genes link both phenotypes. J Bacteriol. 1991 Feb;173(3):1230–1240. doi: 10.1128/jb.173.3.1230-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T., Cowan S. W. Prediction of membrane-spanning beta-strands and its application to maltoporin. Protein Sci. 1993 Aug;2(8):1361–1363. doi: 10.1002/pro.5560020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K., Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Schulz G. E. Structure of porin refined at 1.8 A resolution. J Mol Biol. 1992 Sep 20;227(2):493–509. doi: 10.1016/0022-2836(92)90903-w. [DOI] [PubMed] [Google Scholar]
- Willems R. J., van der Heide H. G., Mooi F. R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992 Sep;6(18):2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K. Genetics of adhesive fimbriae of intestinal Escherichia coli. Curr Top Microbiol Immunol. 1990;151:29–53. doi: 10.1007/978-3-642-74703-8_2. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]