Abstract
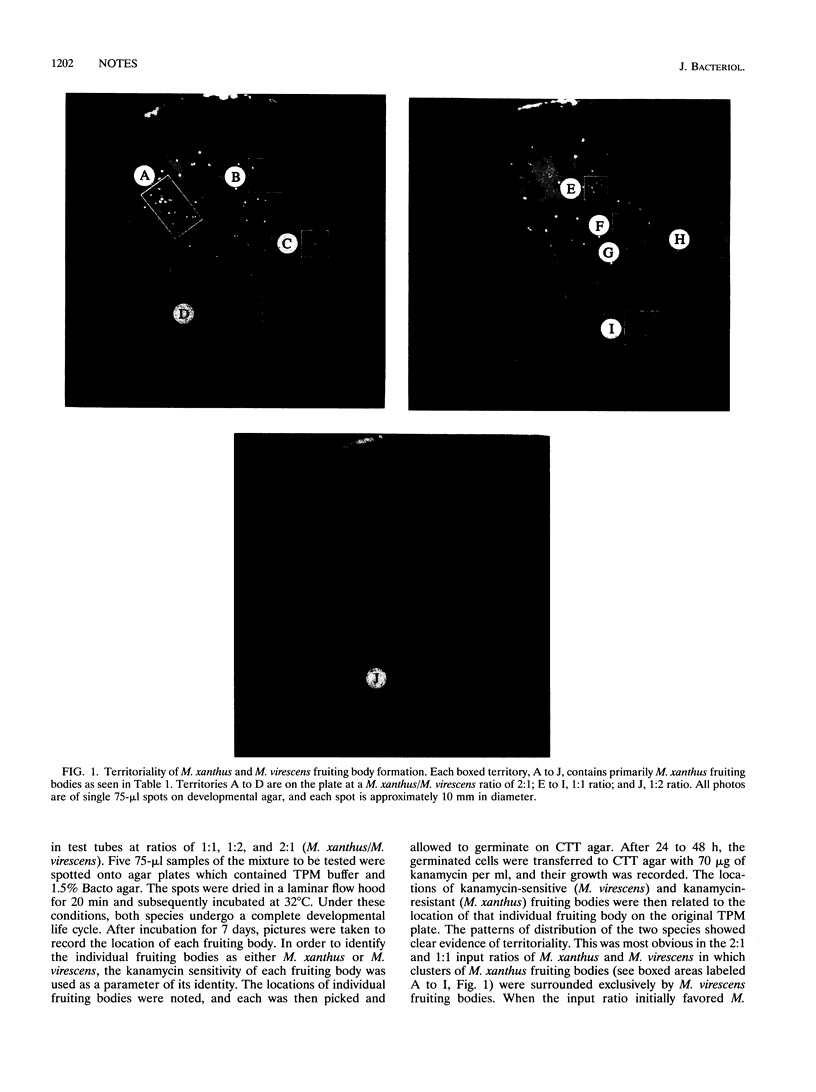
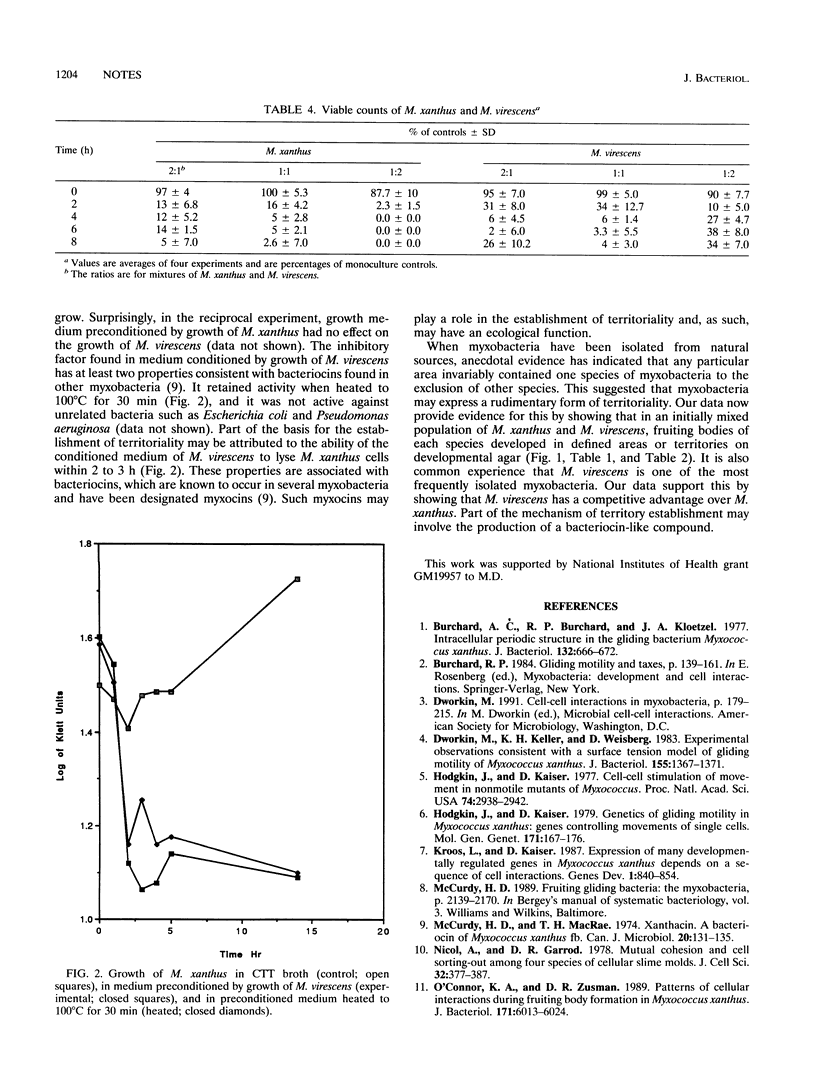
It is unusual to find fruiting bodies of different myxobacteria occupying the same territory on natural samples. We were thus interested in determining whether myxobacteria establish territorial dominance and, if so, what the mechanism of that interaction is. We had previously observed that vegetative swarms of Myxococcus xanthus and Stigmatella aurantiaca placed close to each other on an agar surface initially merged but eventually separated. Further studies indicated that these two species also formed separate fruiting bodies when mixed together on developmental agar (unpublished observation). We examined the interactions between two more closely related myxobacteria, M. xanthus and M. virescens, in greater detail. When mixtures of a kanamycin-resistant strain of M. xanthus and a kanamycin-sensitive strain of M. virescens were placed together under developmental conditions, the cells sorted themselves out and established separate fruiting body territories. In addition, differential viable counts of a mixture of the two species during development indicated that each strain was producing an extracellular component that inhibited the growth and development of the other. Nevertheless, finally, M. virescens invariably outcompeted M. xanthus at all input ratios of M. xanthus/M. virescens tested. This is consistent with the observation that M. virescens is by far the more commonly encountered of the two species. The properties of the inhibitory substance from M. virescens are consistent with the possibility that it is a bacteriocin. Our working hypothesis is that the bacteriocin plays a role in the establishment of myxobacterial territoriality. If so, this is an example of an ecological function of bacteriocins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchard A. C., Burchard R. P., Kloetzel J. A. Intracellular, periodic structures in the gliding bacterium Myxococcus xanthus. J Bacteriol. 1977 Nov;132(2):666–672. doi: 10.1128/jb.132.2.666-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M., Keller K. H., Weisberg D. Experimental observations consistent with a surface tension model of gliding motility of Myxococcus xanthus. J Bacteriol. 1983 Sep;155(3):1367–1371. doi: 10.1128/jb.155.3.1367-1371.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1987 Oct;1(8):840–854. doi: 10.1101/gad.1.8.840. [DOI] [PubMed] [Google Scholar]
- McCurdy H. D., Jr, MacRae T. H. Xanthacin. A bacteriocin of Myxococcus xanthus fb. Can J Microbiol. 1974 Feb;20(2):131–135. doi: 10.1139/m74-021. [DOI] [PubMed] [Google Scholar]
- Nicol A., Garrod D. R. Mutual cohesion and cell sorting-out among four species of cellular slime moulds. J Cell Sci. 1978 Aug;32:377–387. doi: 10.1242/jcs.32.1.377. [DOI] [PubMed] [Google Scholar]
- O'Connor K. A., Zusman D. R. Patterns of cellular interactions during fruiting-body formation in Myxococcus xanthus. J Bacteriol. 1989 Nov;171(11):6013–6024. doi: 10.1128/jb.171.11.6013-6024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L. J., Dodd J., Barondes S. H., Jessell T. M. Selective expression of endogenous lactose-binding lectins and lactoseries glycoconjugates in subsets of rat sensory neurons. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2248–2252. doi: 10.1073/pnas.83.7.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Nir R., Sahar E., Rosenberg E. Cell-density-dependent lysis and sporulation of Myxococcus xanthus in agarose microbeads. J Bacteriol. 1989 Sep;171(9):4923–4929. doi: 10.1128/jb.171.9.4923-4929.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. N., Kilsby D. C. A rapid, inexpensive bacterial count technique using agar droplets. J Appl Bacteriol. 1971 Jun;34(2):435–440. doi: 10.1111/j.1365-2672.1971.tb02303.x. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld J. Evidence for differential cellular adhesion as the mechanism of sorting-out of various cellular slime mold species. J Embryol Exp Morphol. 1979 Oct;53:163–178. [PubMed] [Google Scholar]




