Abstract
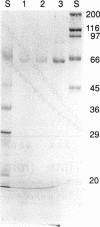
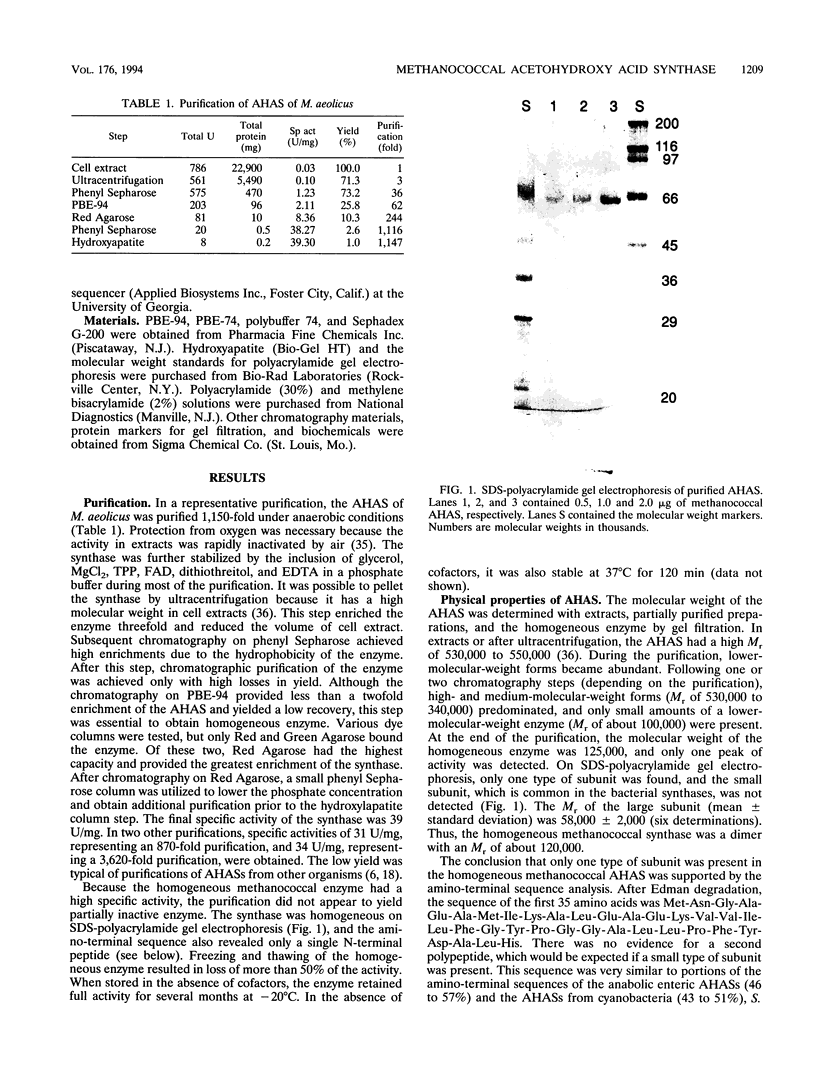
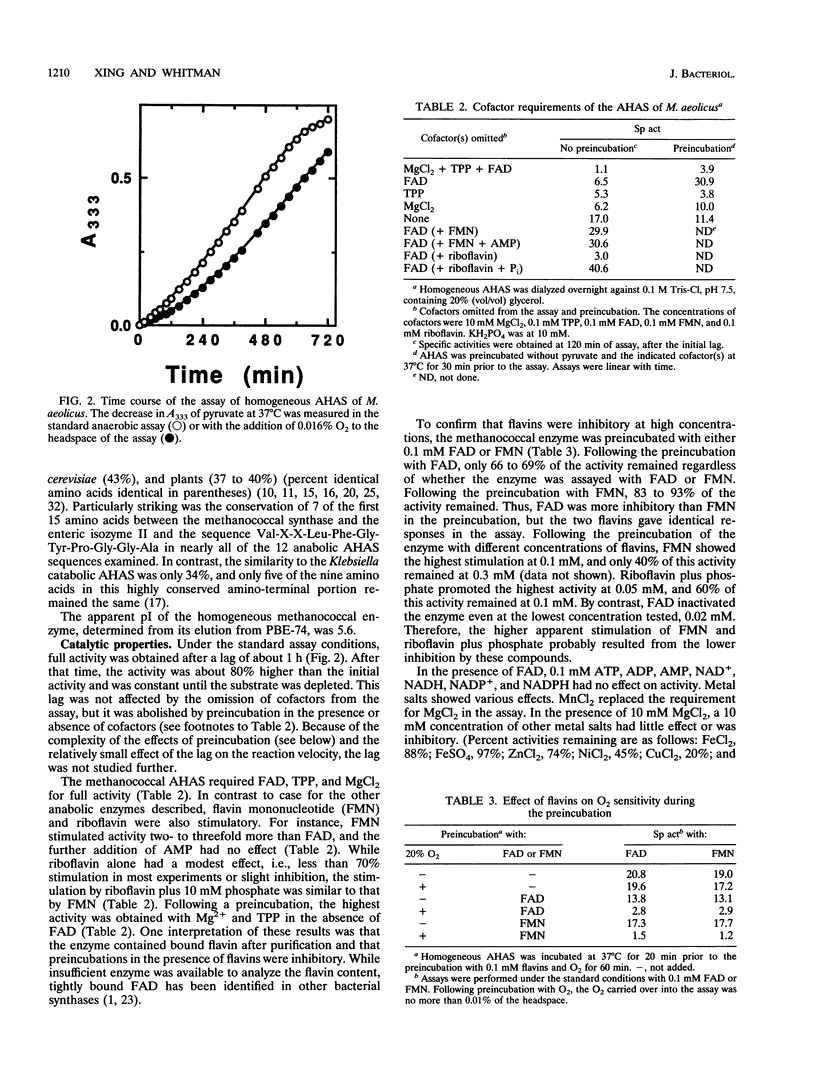
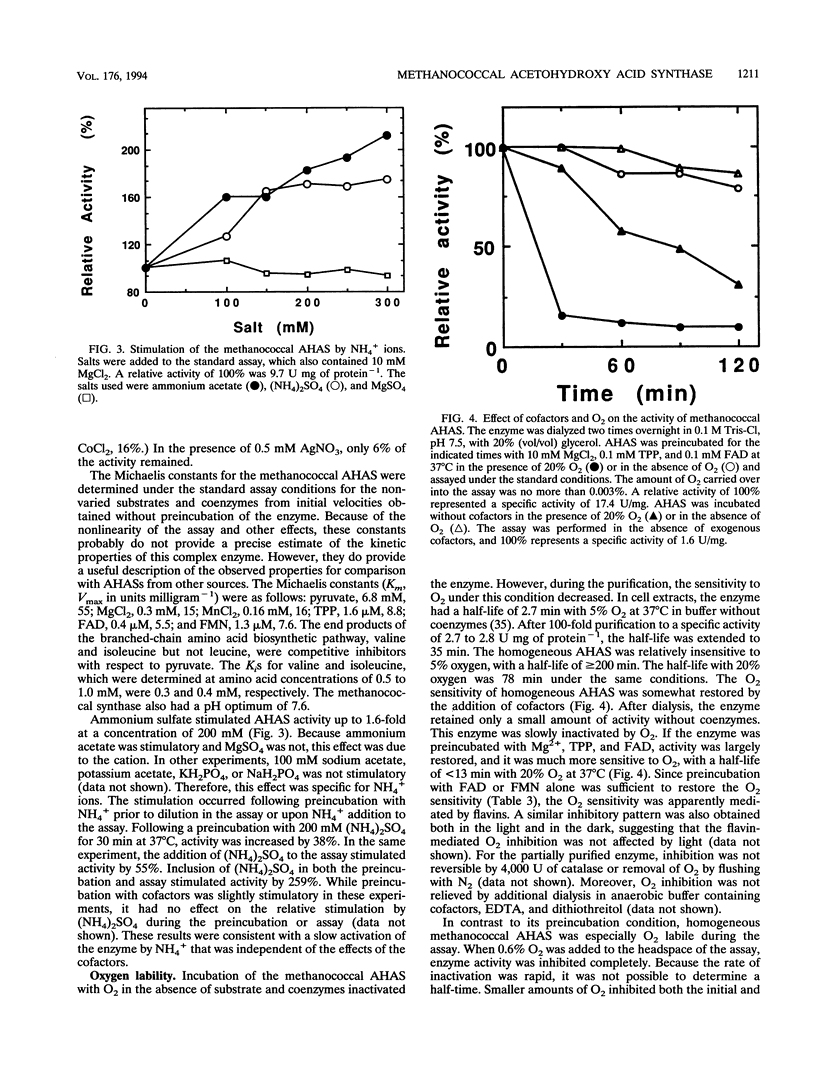
Acetohydroxy acid synthase (EC 4.1.3.18) of the archaebacterium Methanococcus aeolicus was purified 1,150-fold to homogeneity. The molecular weight of the purified enzyme was 125,000, and it contained only one type of subunit (M(r) = 58,000). The amino-terminal sequence had 46 to 57% similarity to those of the large subunits of the eubacterial anabolic enzymes and 37 to 43% similarity to those of the yeast and plant enzymes. The methanococcal enzyme had a pH optimum of 7.6. The pI, estimated by chromatofocusing, was 5.6. Activity required Mg2+ or Mn2+ ions, thiamine pyrophosphate, and a flavin. Flavin adenine dinucleotide, flavin mononucleotide, and riboflavin plus 10 mM phosphate all supported activity. However, activity was strongly inhibited by these flavins at 0.3 mM. The Michaelis constants for pyruvate, MgCl2, MnCl2, thiamine pyrophosphate, flavin adenine dinucleotide, and flavin mononucleotide were 6.8 mM, 0.3 mM, 0.16 mM, 1.6 microM, 0.4 microM, and 1.3 microM, respectively. In cell extracts, the enzyme was sensitive to O2 (half-life = 2.7 min with 5% O2 in the headspace), but the purified enzyme was less sensitive to O2 (half-life = 78.0 min with 20% O2). Reconstitution of the enzyme with flavin adenine dinucleotide increased the sensitivity to O2. Moreover, in the assay the homogeneous enzyme was rapidly inactivated by O2, and the concentration required for 50% inhibition (I50) was obtained with an atmosphere of 0.11% O2. The methanococcal enzyme has similarities to the eubacterial and eucaryotic enzymes, consistent with the ancient origin of the archaebacterial enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Koziell D. A. Acetolactate synthase of Pseudomonas aeruginosa I. Purification and allosteric properties. Biochim Biophys Acta. 1973 Sep 15;321(1):348–355. doi: 10.1016/0005-2744(73)90089-2. [DOI] [PubMed] [Google Scholar]
- Arfin S. M., Koziell D. A. Acetolactate synthase of Pseudomonas aeruginosa. II. Evidence for the presence of two nonidentical subunits. Biochim Biophys Acta. 1973 Sep 15;321(1):356–360. doi: 10.1016/0005-2744(73)90090-9. [DOI] [PubMed] [Google Scholar]
- Barak Z., Calvo J. M., Schloss J. V. Acetolactate synthase isozyme III from Escherichia coli. Methods Enzymol. 1988;166:455–458. doi: 10.1016/s0076-6879(88)66059-9. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E., Jr Common ancestry of Escherichia coli pyruvate oxidase and the acetohydroxy acid synthases of the branched-chain amino acid biosynthetic pathway. J Bacteriol. 1988 Sep;170(9):3937–3945. doi: 10.1128/jb.170.9.3937-3945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J., Böger P. Oligomeric forms of plant acetolactate synthase depend on flavin adenine dinucleotide. Plant Physiol. 1990 Jul;93(3):1027–1031. doi: 10.1104/pp.93.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Role of small subunit (IlvN polypeptide) of acetohydroxyacid synthase I from Escherichia coli K-12 in sensitivity of the enzyme to valine inhibition. J Bacteriol. 1986 Jun;166(3):901–904. doi: 10.1128/jb.166.3.901-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabau C., Cronan J. E., Jr Nucleotide sequence and deduced amino acid sequence of Escherichia coli pyruvate oxidase, a lipid-activated flavoprotein. Nucleic Acids Res. 1986 Jul 11;14(13):5449–5460. doi: 10.1093/nar/14.13.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F. Degradative acetolactate synthase of Bacillus subtilis: purification and properties. J Bacteriol. 1975 Mar;121(3):917–922. doi: 10.1128/jb.121.3.917-922.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano A., De Rossi E., Zanaria E., Barbierato L., Ciferri O., Riccardi G. Molecular characterization of the genes encoding acetohydroxy acid synthase in the cyanobacterium Spirulina platensis. J Gen Microbiol. 1992 Jul;138(7):1399–1408. doi: 10.1099/00221287-138-7-1399. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Wang P. Y., Wu C. M., Hwang D. C., Chang H. Y. Cloning, sequencing and heterologous expression of a Klebsiella pneumoniae gene encoding an FAD-independent acetolactate synthase. Gene. 1992 Aug 1;117(1):125–130. doi: 10.1016/0378-1119(92)90500-o. [DOI] [PubMed] [Google Scholar]
- Poulsen C., Stougaard P. Purification and properties of Saccharomyces cerevisiae acetolactate synthase from recombinant Escherichia coli. Eur J Biochem. 1989 Nov 6;185(2):433–439. doi: 10.1111/j.1432-1033.1989.tb15133.x. [DOI] [PubMed] [Google Scholar]
- Ricca E., Limauro D., Lago C. T., de Felice M. Enhanced acetohydroxy acid synthase III activity in an ilvH mutant of Escherichia coli K-12. J Bacteriol. 1988 Nov;170(11):5197–5199. doi: 10.1128/jb.170.11.5197-5199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge R. G., Quellet T., Hattori J., Miki B. L. Molecular characterization and genetic origin of the Brassica napus acetohydroxyacid synthase multigene family. Mol Gen Genet. 1991 Sep;229(1):31–40. doi: 10.1007/BF00264210. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Snoep J. L., Teixeira de Mattos M. J., Starrenburg M. J., Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992 Jul;174(14):4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer F. C. 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol. 1975;41:518–532. doi: 10.1016/s0076-6879(75)41108-9. [DOI] [PubMed] [Google Scholar]
- Störmer F. C. Isolation of crystalline pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. J Biol Chem. 1967 Apr 25;242(8):1756–1759. [PubMed] [Google Scholar]
- Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. II. Evidence that it is not a flavoprotein. J Biol Chem. 1968 Jul 10;243(13):3740–3741. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weinstock O., Sella C., Chipman D. M., Barak Z. Properties of subcloned subunits of bacterial acetohydroxy acid synthases. J Bacteriol. 1992 Sep;174(17):5560–5566. doi: 10.1128/jb.174.17.5560-5566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Sohn S., Kuk S., Xing R. Role of Amino Acids and Vitamins in Nutrition of Mesophilic Methanococcus spp. Appl Environ Microbiol. 1987 Oct;53(10):2373–2378. doi: 10.1128/aem.53.10.2373-2378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Characterization of amino acid aminotransferases of Methanococcus aeolicus. J Bacteriol. 1992 Jan;174(2):541–548. doi: 10.1128/jb.174.2.541-548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Characterization of enzymes of the branched-chain amino acid biosynthetic pathway in Methanococcus spp. J Bacteriol. 1991 Mar;173(6):2086–2092. doi: 10.1128/jb.173.6.2086-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Sulfometuron methyl-sensitive and -resistant acetolactate synthases of the archaebacteria Methanococcus spp. J Bacteriol. 1987 Oct;169(10):4486–4492. doi: 10.1128/jb.169.10.4486-4492.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]