Abstract
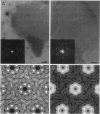
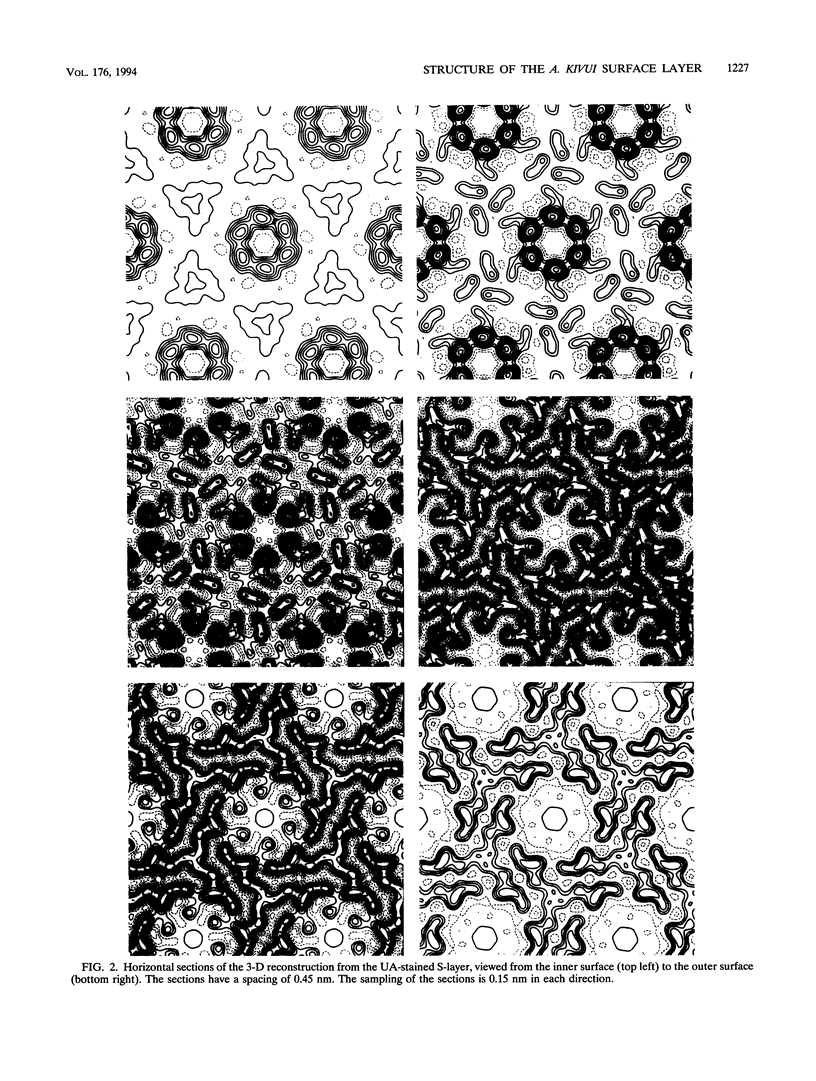
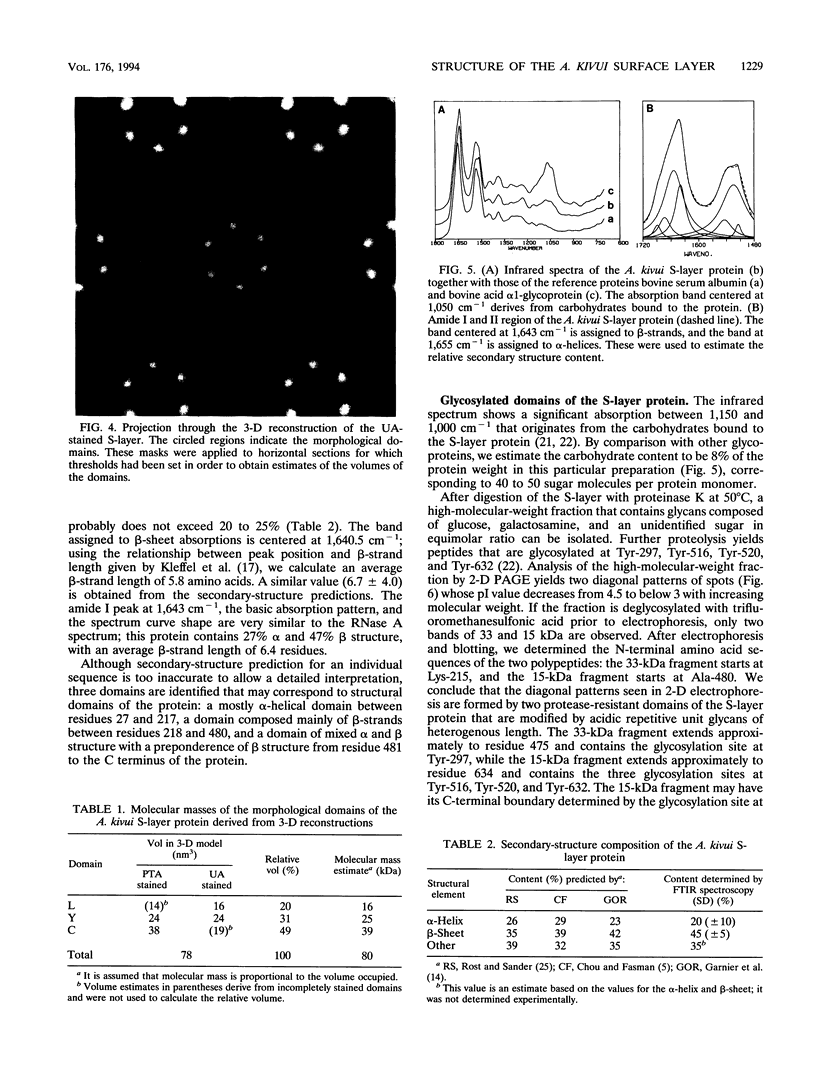
The three-dimensional structure of the Acetogenium kivui surface layer (S-layer) has been determined to a resolution of 1.7 nm by electron crystallographic techniques. Two independent reconstructions were made from layers negatively stained with uranyl acetate and Na-phosphotungstate. The S-layer has p6 symmetry with a center-to-center spacing of approximately 19 nm. Within the layer, six monomers combine to form a ring-shaped core surrounded by a fenestrated rim and six spokes that point towards the axis of threefold symmetry and provide lateral connectivity to other hexamers in the layer. The structure of the A. kivui S-layer protein is very similar to that of the Bacillus brevis middle wall protein, with which it shares an N-terminal domain of homology. This domain is found in several other extracellular proteins, including the S-layer proteins from Bacillus sphaericus and Thermus thermophilus, Omp alpha from Thermotoga maritima, an alkaline cellulase from Bacillus strain KSM-635, and xylanases from Clostridium thermocellum and Thermoanaerobacter saccharolyticum, and may serve to anchor these proteins to the peptidoglycan. To our knowledge, this is the first example of a domain conserved in several S-layer proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Engelhardt H., Baumeister W., Kay W. W., Trust T. J. Three-dimensional structure of an open form of the surface layer from the fish pathogen Aeromonas salmonicida. J Bacteriol. 1989 Jan;171(1):190–197. doi: 10.1128/jb.171.1.190-197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Tsuboi A., Takagi H., Naruse Y., Yamagata H., Tsukagoshi N., Udaka S. Conserved structures of cell wall protein genes among protein-producing Bacillus brevis strains. J Bacteriol. 1990 Mar;172(3):1312–1320. doi: 10.1128/jb.172.3.1312-1320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. M., Cejka Z., Lupas A., Lottspeich F., Baumeister W. Isolation and cloning of Omp alpha, a coiled-coil protein spanning the periplasmic space of the ancestral eubacterium Thermotoga maritima. EMBO J. 1992 Dec;11(12):4369–4378. doi: 10.1002/j.1460-2075.1992.tb05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H., Saxton W. O., Baumeister W. Three-dimensional structure of the tetragonal surface layer of Sporosarcina ureae. J Bacteriol. 1986 Oct;168(1):309–317. doi: 10.1128/jb.168.1.309-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T., Béguin P., Aubert J. P. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J Bacteriol. 1993 Apr;175(7):1891–1899. doi: 10.1128/jb.175.7.1891-1899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Lüthy R., Eisenberg D. Profile analysis. Methods Enzymol. 1990;183:146–159. doi: 10.1016/0076-6879(90)83011-w. [DOI] [PubMed] [Google Scholar]
- Hastie A. T., Brinton C. C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979 Jun;138(3):1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Baumeister W. S-layer protein gene of Acetogenium kivui: cloning and expression in Escherichia coli and determination of the nucleotide sequence. J Bacteriol. 1989 Nov;171(11):6307–6315. doi: 10.1128/jb.171.11.6307-6315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Rudolf S., Oschkinat H., Mengele R., Sumper M., Kellermann J., Lottspeich F., Baumeister W. Evidence for tyrosine-linked glycosaminoglycan in a bacterial surface protein. Biol Chem Hoppe Seyler. 1992 Apr;373(4):171–176. doi: 10.1515/bchm3.1992.373.1.171. [DOI] [PubMed] [Google Scholar]
- Phipps B. M., Huber R., Baumeister W. The cell envelope of the hyperthermophilic archaebacterium Pyrobaculum organotrphum consists of two regularly arrayed protein layers: three-dimensional structure of the outer layer. Mol Microbiol. 1991 Feb;5(2):253–265. doi: 10.1111/j.1365-2958.1991.tb02106.x. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993 Jul 20;232(2):584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. Principles of organization in S layers. J Mol Biol. 1986 Jan 20;187(2):251–253. doi: 10.1016/0022-2836(86)90232-9. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi A., Engelhardt H., Santarius U., Tsukagoshi N., Udaka S., Baumeister W. Three-dimensional structure of the surface protein layer (MW layer) of Bacillus brevis 47. J Ultrastruct Mol Struct Res. 1989 Aug;102(2):178–187. doi: 10.1016/0889-1605(89)90055-4. [DOI] [PubMed] [Google Scholar]