Abstract
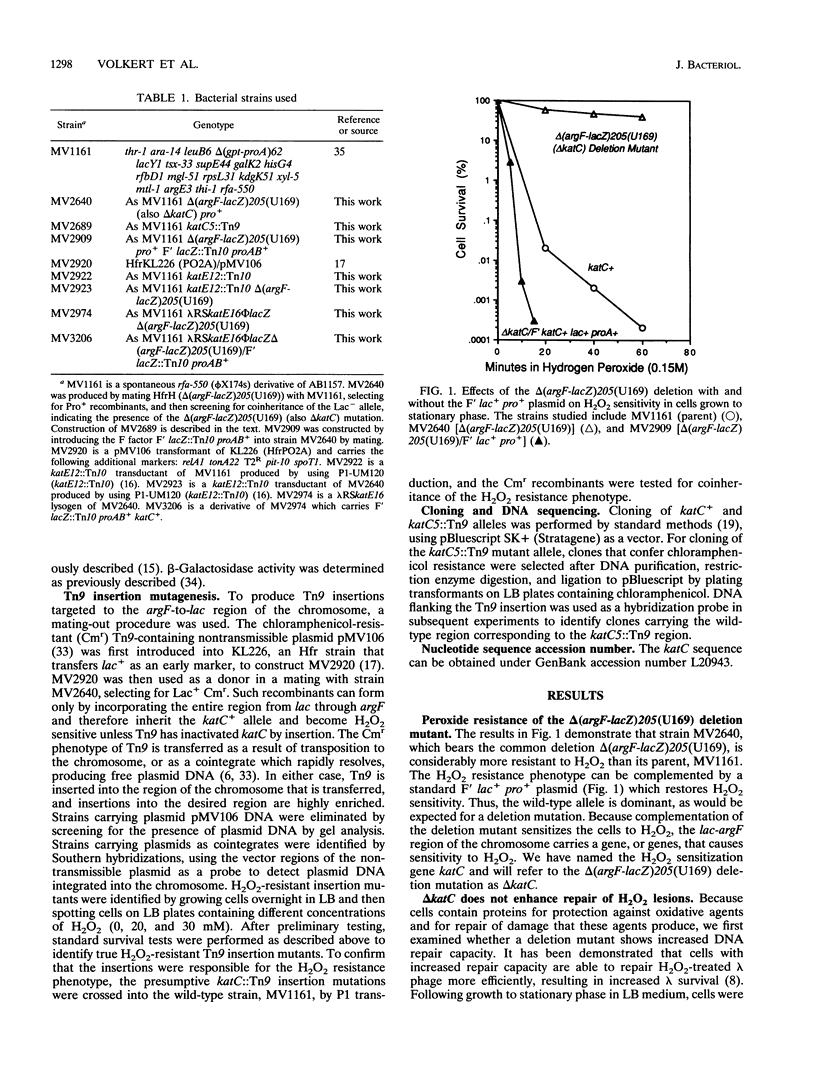
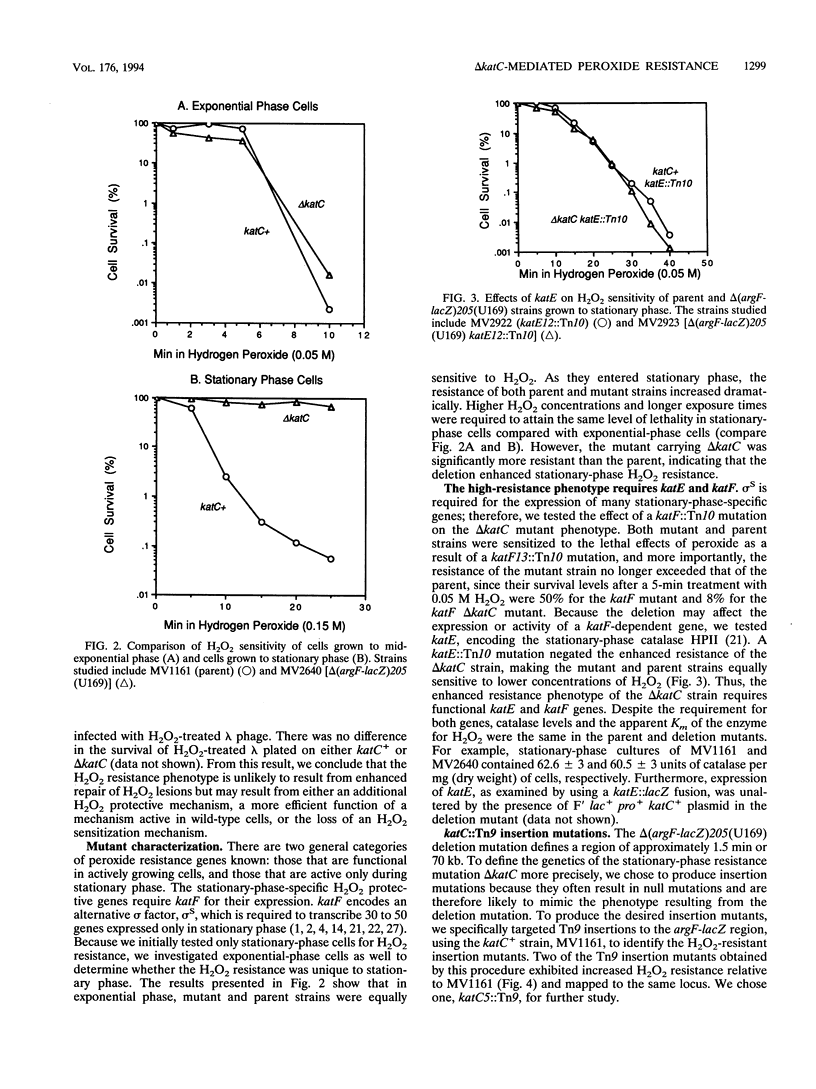
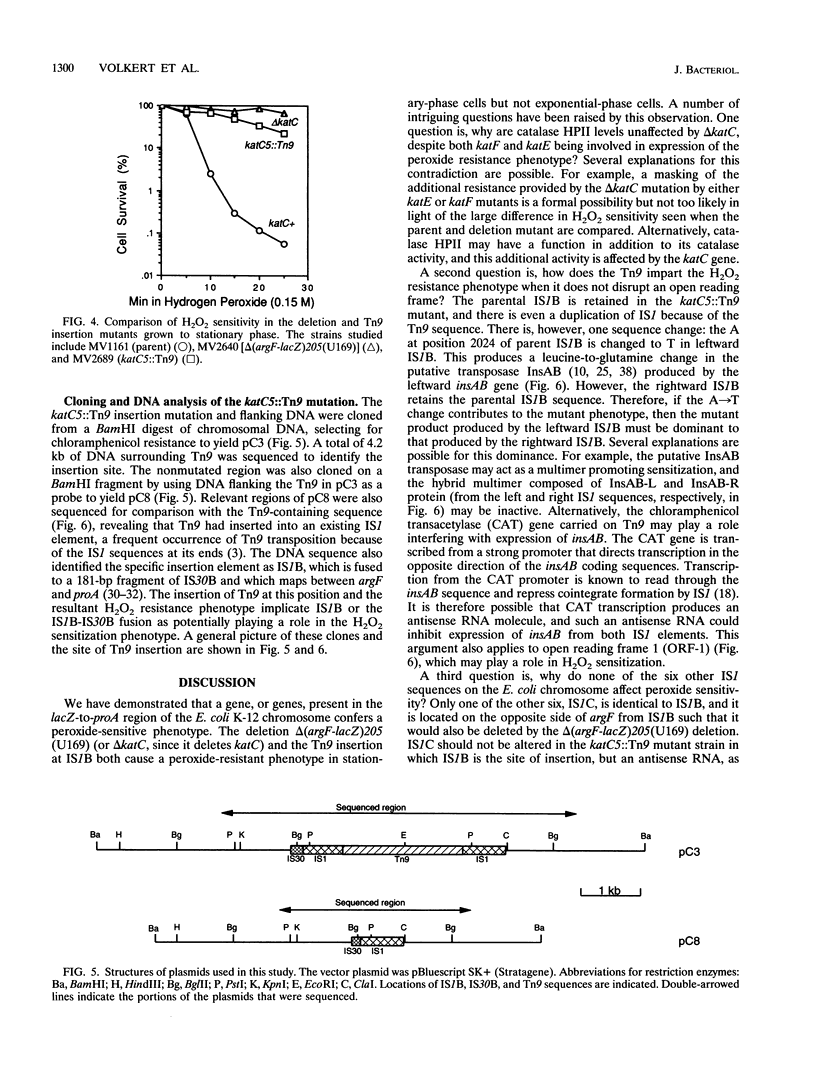
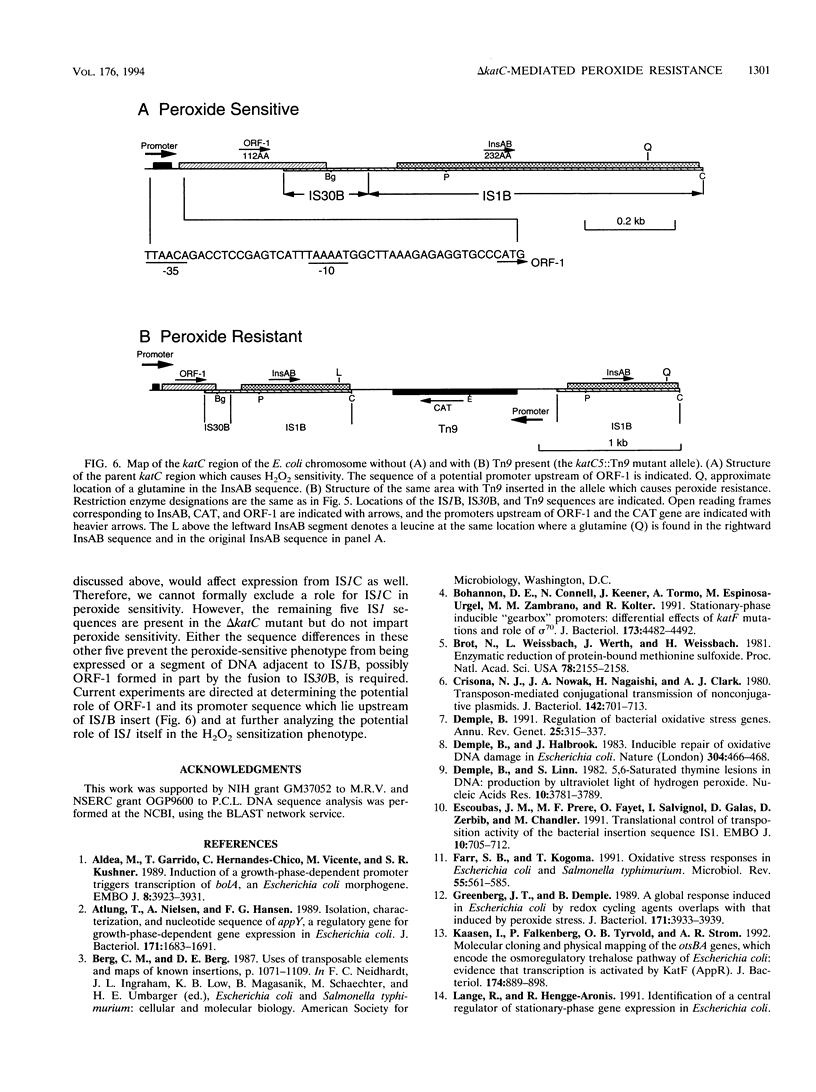
In this study, we demonstrate that a strain bearing the delta (argF-lacZ)205(U169) deletion exhibits a high level of resistance to hydrogen peroxide compared with its undeleted parent. Our initial investigation of the mechanism behind the observed differences in peroxide resistance when parent and mutant strains are compared indicates that the parent strain carries a region near argF that is responsible for the H2O2-sensitive phenotype, which we have named katC. The H2O2 resistance phenotype of the delta katC [delta (argF-lacZ)205(U169)] mutant strain can be duplicated by Tn9 insertion in a specific locus (katC5::Tn9) which maps near argF. The increased H2O2 resistance of the delta katC and katC5::Tn9 mutant strains can be seen only when cells are grown to stationary phase; exponential-phase cells are unaffected by the presence or absence of katC. This H2O2 resistance mechanism requires functional katE and katF genes, which suggests that the mechanism of H2O2 resistance may involve the activity of the stationary-phase-specific catalase HPII. Cloning, DNA sequencing, and analysis of the katC5::Tn9 insertion allele in comparison with its parent allele implicate two insertion elements, IS1B and IS30B, and suggest that their presence sensitizes parent cells to H2O2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Garrido T., Hernández-Chico C., Vicente M., Kushner S. R. Induction of a growth-phase-dependent promoter triggers transcription of bolA, an Escherichia coli morphogene. EMBO J. 1989 Dec 1;8(12):3923–3931. doi: 10.1002/j.1460-2075.1989.tb08573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T., Nielsen A., Hansen F. G. Isolation, characterization, and nucleotide sequence of appY, a regulatory gene for growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1989 Mar;171(3):1683–1691. doi: 10.1128/jb.171.3.1683-1691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon D. E., Connell N., Keener J., Tormo A., Espinosa-Urgel M., Zambrano M. M., Kolter R. Stationary-phase-inducible "gearbox" promoters: differential effects of katF mutations and role of sigma 70. J Bacteriol. 1991 Jul;173(14):4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Weissbach L., Werth J., Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisona N. J., Nowak J. A., Nagaishi H., Clark A. J. Transposon-mediated conjugational transmission of nonconjugative plasmids. J Bacteriol. 1980 May;142(2):701–713. doi: 10.1128/jb.142.2.701-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet. 1991;25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- Escoubas J. M., Prère M. F., Fayet O., Salvignol I., Galas D., Zerbib D., Chandler M. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 1991 Mar;10(3):705–712. doi: 10.1002/j.1460-2075.1991.tb08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasen I., Falkenberg P., Styrvold O. B., Strøm A. R. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (AppR) J Bacteriol. 1992 Feb;174(3):889–898. doi: 10.1128/jb.174.3.889-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Switala J. Multiple catalases in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3601–3607. doi: 10.1128/jb.169.8.3601-3607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Switala J., Triggs-Raine B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985 Nov 15;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- Machida C., Machida Y., Wang H. C., Ishizaki K., Ohtsubo E. Repression of cointegration ability of insertion element IS1 by transcriptional readthrough from flanking regions. Cell. 1983 Aug;34(1):135–142. doi: 10.1016/0092-8674(83)90143-5. [DOI] [PubMed] [Google Scholar]
- Mulvey M. R., Loewen P. C. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén A., Arnqvist A., Hammar M., Sukupolvi S., Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993 Feb;7(4):523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Sak B. D., Eisenstark A., Touati D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc Natl Acad Sci U S A. 1989 May;86(9):3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhorn H. E., Stones V. L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992 Jul;174(14):4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y., Ohtsubo E. DNA sequences required for translational frameshifting in production of the transposase encoded by IS1. Mol Gen Genet. 1992 Nov;235(2-3):325–332. doi: 10.1007/BF00279377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Takayanagi Y., Fujita N., Ishihama A., Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati E., Dassa E., Dassa J., Boquet P. L., Touati D. Are appR and katF the same Escherichia coli gene encoding a new sigma transcription initiation factor? Res Microbiol. 1991 Jan;142(1):29–36. doi: 10.1016/0923-2508(91)90094-q. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Four types of IS1 with differences in nucleotide sequence reside in the Escherichia coli K-12 chromosome. Gene. 1991 Feb 1;98(1):1–5. doi: 10.1016/0378-1119(91)90096-t. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion element IS30 in the Escherichia coli K12 chromosome. Mol Gen Genet. 1990 Jul;222(2-3):317–322. doi: 10.1007/BF00633835. [DOI] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion elements IS1, IS2 and IS3 on the Escherichia coli K-12 chromosome. Role of the insertion elements in formation of Hfrs and F' factors and in rearrangement of bacterial chromosomes. J Mol Biol. 1989 Aug 20;208(4):601–614. doi: 10.1016/0022-2836(89)90151-4. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Hajec L. I. Molecular analysis of the aidD6::Mu d1 (bla lac) fusion mutation of Escherichia coli K12. Mol Gen Genet. 1991 Oct;229(2):319–323. doi: 10.1007/BF00272173. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Hajec L. I., Nguyen D. C. Induction of the alkylation-inducible aidB gene of Escherichia coli by anaerobiosis. J Bacteriol. 1989 Feb;171(2):1196–1198. doi: 10.1128/jb.171.2.1196-1198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Nguyen D. C. Induction of specific Escherichia coli genes by sublethal treatments with alkylating agents. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4110–4114. doi: 10.1073/pnas.81.13.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbib D., Polard P., Escoubas J. M., Galas D., Chandler M. The regulatory role of the IS1-encoded InsA protein in transposition. Mol Microbiol. 1990 Mar;4(3):471–477. doi: 10.1111/j.1365-2958.1990.tb00613.x. [DOI] [PubMed] [Google Scholar]
- von Ossowski I., Mulvey M. R., Leco P. A., Borys A., Loewen P. C. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol. 1991 Jan;173(2):514–520. doi: 10.1128/jb.173.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


