Abstract
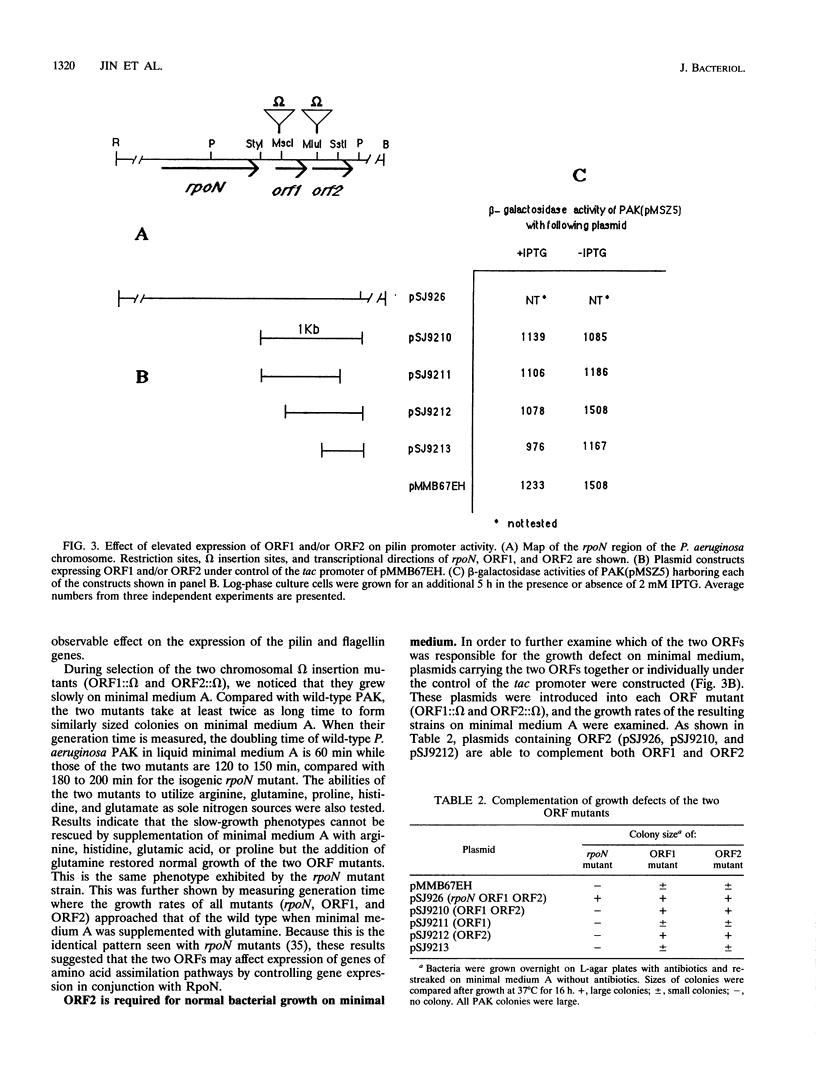
The rpoN gene of Pseudomonas aeruginosa is required for the expression of a number of diverse genes, ranging from several classes of bacterial adhesins to enzymes for amino acid biosynthesis. The nucleotide sequence of the rpoN gene and its flanking region has been determined. The deduced amino acid sequence of the rpoN product is highly homologous to sequences of RpoN proteins of other microorganisms. Moreover, two open reading frames (ORF1 and ORF2) encoding peptides of 103 and 154 amino acids long, respectively, were found downstream of the rpoN gene. These two ORF products have a high degree of amino acid sequence homology with products of similar ORFs located adjacent to the rpoN genes in other microorganisms. Mutations in either ORF lead to a significant increase in P. aeruginosa generation time when propagated on minimal medium. These mutations had no effect on the expression of pilin or flagellin genes, whose expression depends on RpoN. Complementation analysis showed that the two ORFs are in the same transcriptional unit and the growth defects of the two ORF mutants on minimal medium are due to mutational effects on ORF2. The adverse effect of the ORF mutations on the growth of P. aeruginosa in minimal media can be suppressed by the addition of glutamine but not arginine, glutamate, histidine, or proline. Since rpoN mutants of P. aeruginosa display this same amino acid requirement for growth, the ORF2 product very likely functions as a coinducer of some but not all of the RpoN-controlled genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger D. K., Woods D. R., Rawlings D. E. Complementation of Escherichia coli sigma 54 (NtrA)-dependent formate hydrogenlyase activity by a cloned Thiobacillus ferrooxidans ntrA gene. J Bacteriol. 1990 Aug;172(8):4399–4406. doi: 10.1128/jb.172.8.4399-4406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Pasloske B. L., Paranchych W. Expression of the Pseudomonas aeruginosa PAK pilin gene in Escherichia coli. J Bacteriol. 1986 Feb;165(2):625–630. doi: 10.1128/jb.165.2.625-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Geerse R. H., van der Pluijm J., Postma P. W. The repressor of the PEP:fructose phosphotransferase system is required for the transcription of the pps gene of Escherichia coli. Mol Gen Genet. 1989 Aug;218(2):348–352. doi: 10.1007/BF00331288. [DOI] [PubMed] [Google Scholar]
- Gillen K. L., Hughes K. T. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol. 1991 Apr;173(7):2301–2310. doi: 10.1128/jb.173.7.2301-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Hobbs M., Collie E. S., Free P. D., Livingston S. P., Mattick J. S. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993 Mar;7(5):669–682. doi: 10.1111/j.1365-2958.1993.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Inouye S., Yamada M., Nakazawa A., Nakazawa T. Cloning and sequence analysis of the ntrA (rpoN) gene of Pseudomonas putida. Gene. 1989 Dec 21;85(1):145–152. doi: 10.1016/0378-1119(89)90474-5. [DOI] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J Bacteriol. 1992 Jun;174(11):3514–3521. doi: 10.1128/jb.174.11.3514-3521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Haselkorn R. The DNA sequence of the Rhodobacter capsulatus ntrA, ntrB and ntrC gene analogues required for nitrogen fixation. Mol Gen Genet. 1989 Feb;215(3):507–516. doi: 10.1007/BF00427050. [DOI] [PubMed] [Google Scholar]
- Kullik I., Fritsche S., Knobel H., Sanjuan J., Hennecke H., Fischer H. M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the sigma 54 gene (rpoN). J Bacteriol. 1991 Feb;173(3):1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J., Coppard J. R. Mutations in genes downstream of the rpoN gene (encoding sigma 54) of Klebsiella pneumoniae affect expression from sigma 54-dependent promoters. Mol Microbiol. 1989 Dec;3(12):1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Merrick M. J., Gibbins J. R. The nucleotide sequence of the nitrogen-regulation gene ntrA of Klebsiella pneumoniae and comparison with conserved features in bacterial RNA polymerase sigma factors. Nucleic Acids Res. 1985 Nov 11;13(21):7607–7620. doi: 10.1093/nar/13.21.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M., Gibbins J., Toukdarian A. The nucleotide sequence of the sigma factor gene ntrA (rpoN) of Azotobacter vinelandii: analysis of conserved sequences in NtrA proteins. Mol Gen Genet. 1987 Dec;210(2):323–330. doi: 10.1007/BF00325701. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J., Mullin D. A., Ramakrishnan G., Newton A. Escherichia coli sigma 54 RNA polymerase recognizes Caulobacter crescentus flbG and flaN flagellar gene promoters in vitro. J Bacteriol. 1989 Jan;171(1):383–391. doi: 10.1128/jb.171.1.383-391.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D., Bergman S., Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990 Jun;172(6):2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K., Kutsukake K., Suzuki H., Lino T. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an antisigma factor inhibits the activity of the flagellum-specific sigma factor, sigma F. Mol Microbiol. 1992 Nov;6(21):3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- Orsini G., Ouhammouch M., Le Caer J. P., Brody E. N. The asiA gene of bacteriophage T4 codes for the anti-sigma 70 protein. J Bacteriol. 1993 Jan;175(1):85–93. doi: 10.1128/jb.175.1.85-93.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasloske B. L., Finlay B. B., Paranchych W. Cloning and sequencing of the Pseudomonas aeruginosa PAK pilin gene. FEBS Lett. 1985 Apr 22;183(2):408–412. doi: 10.1016/0014-5793(85)80821-8. [DOI] [PubMed] [Google Scholar]
- Popham D., Keener J., Kustu S. Purification of the alternative sigma factor, sigma 54, from Salmonella typhimurium and characterization of sigma 54-holoenzyme. J Biol Chem. 1991 Oct 15;266(29):19510–19518. [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Albright L. M., Ausubel F. M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987 Jun;169(6):2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. A., Ramphal R., Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992 Sep;60(9):3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986 Feb;165(2):367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny B., Hennecke H. The -24/-12 promoter comes of age. FEMS Microbiol Rev. 1989 Dec;5(4):341–357. doi: 10.1016/0168-6445(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Totten P. A., Lara J. C., Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990 Jan;172(1):389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990 Dec;172(12):7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrelmann J., Eitinger M., Schwartz E., Römermann D., Friedrich B. Nucleotide sequence of the rpoN (hno) gene region of Alcaligenes eutrophus: evidence for a conserved gene cluster. Arch Microbiol. 1992;158(2):107–114. doi: 10.1007/BF00245213. [DOI] [PubMed] [Google Scholar]
- van Slooten J. C., Cervantes E., Broughton W. J., Wong C. H., Stanley J. Sequence and analysis of the rpoN sigma factor gene of rhizobium sp. strain NGR234, a primary coregulator of symbiosis. J Bacteriol. 1990 Oct;172(10):5563–5574. doi: 10.1128/jb.172.10.5563-5574.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]