Abstract
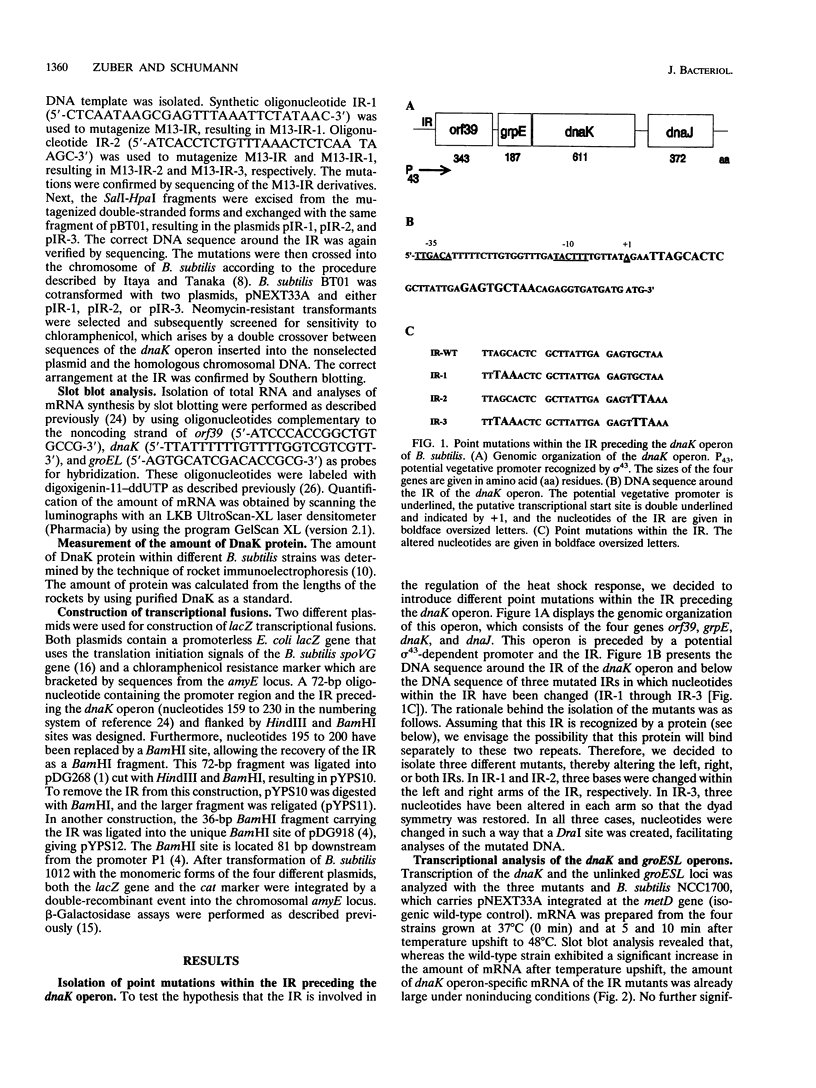
The dnaK and groESL operons of Bacillus subtilis are preceded by a potential sigma 43 promoter sequence (recognized by the vegetative sigma factor) and by an inverted repeat (IR) consisting of 9 bp separated by a 9-bp spacer. Since this IR has been found in many bacterial species, we suspected that it might be involved in heat shock regulation. In order to test this hypothesis, three different mutational alterations of three bases were introduced within the IR preceding the dnaK operon. These mutations were crossed into the chromosome of B. subtilis, and expression of the dnaK and of the unlinked groESL operons was studied. The dnaK operon exhibited increased expression at low temperature and a reduction in the stimulation after temperature upshift. Furthermore, these mutations reduced expression of the groESL operon at low temperature by 50% but did not interfere with stimulation after heat shock. These experiments show that the IR acts as a negative cis element of the dnaK operon. This conclusion was strengthened by the observation that the IR reduced expression of two different transcriptional fusions significantly after its insertion between the promoter and the reporter gene. Since this IR has been described in many bacterial species as preceding only genes of the dnaK and groESL operons, both encoding molecular chaperones (39 cases are documented so far), we designated this heat shock element CIRCE (controlling IR of chaperone expression). Furthermore, we suggest that this novel mechanism is more widespread among eubacteria than the regulation mechanism described for Escherichia coli and has a more ancient origin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniewski C., Savelli B., Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol. 1990 Jan;172(1):86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Singer V. L., Chamberlin M. J. Characterization of heat shock in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1243–1249. doi: 10.1128/jb.168.3.1243-1249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Tréboul G., Karmazyn-Campelli C., Stragier P. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J Mol Biol. 1992 Apr 20;224(4):967–979. doi: 10.1016/0022-2836(92)90463-t. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Straus D. B., Walter W. A., Gross C. A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987 Apr;1(2):179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Gene-directed mutagenesis on the chromosome of Bacillus subtilis 168. Mol Gen Genet. 1990 Sep;223(2):268–272. doi: 10.1007/BF00265063. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Merrill D., Hartley T. F., Claman H. N. Electroimmunodiffusion (EID): a simple, rapid method for quantitation of immunoglobulins in dilute biological fluids. J Lab Clin Med. 1967 Jan;69(1):151–159. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Narberhaus F., Bahl H. Cloning, sequencing, and molecular analysis of the groESL operon of Clostridium acetobutylicum. J Bacteriol. 1992 May;174(10):3282–3289. doi: 10.1128/jb.174.10.3282-3289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F., Giebeler K., Bahl H. Molecular characterization of the dnaK gene region of Clostridium acetobutylicum, including grpE, dnaJ, and a new heat shock gene. J Bacteriol. 1992 May;174(10):3290–3299. doi: 10.1128/jb.174.10.3290-3299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. Construction and properties of Tn917-lac, a transposon derivative that mediates transcriptional gene fusions in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Jan;83(1):140–144. doi: 10.1073/pnas.83.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Shibata T., Ando T. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol Gen Genet. 1979 Feb 26;170(2):117–122. doi: 10.1007/BF00337785. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Schiesswohl M., Völker U., Hecker M., Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön U., Schumann W. Molecular cloning, sequencing, and transcriptional analysis of the groESL operon from Bacillus stearothermophilus. J Bacteriol. 1993 Apr;175(8):2465–2469. doi: 10.1128/jb.175.8.2465-2469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. The heat shock response of E. coli is regulated by changes in the concentration of sigma 32. Nature. 1987 Sep 24;329(6137):348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- Wetzstein M., Schumann W. Promoters of major Escherichia coli heat shock genes seem non-functional in Bacillus subtilis. FEMS Microbiol Lett. 1990 Oct;60(1-2):55–58. doi: 10.1016/0378-1097(90)90344-p. [DOI] [PubMed] [Google Scholar]
- Wetzstein M., Völker U., Dedio J., Löbau S., Zuber U., Schiesswohl M., Herget C., Hecker M., Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992 May;174(10):3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber U., Schumann W. Tn5cos: a transposon for restriction mapping of large plasmids using phage lambda terminase. Gene. 1991 Jul 15;103(1):69–72. doi: 10.1016/0378-1119(91)90392-o. [DOI] [PubMed] [Google Scholar]
- van Asseldonk M., Simons A., Visser H., de Vos W. M., Simons G. Cloning, nucleotide sequence, and regulatory analysis of the Lactococcus lactis dnaJ gene. J Bacteriol. 1993 Mar;175(6):1637–1644. doi: 10.1128/jb.175.6.1637-1644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]