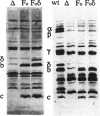
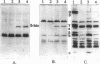
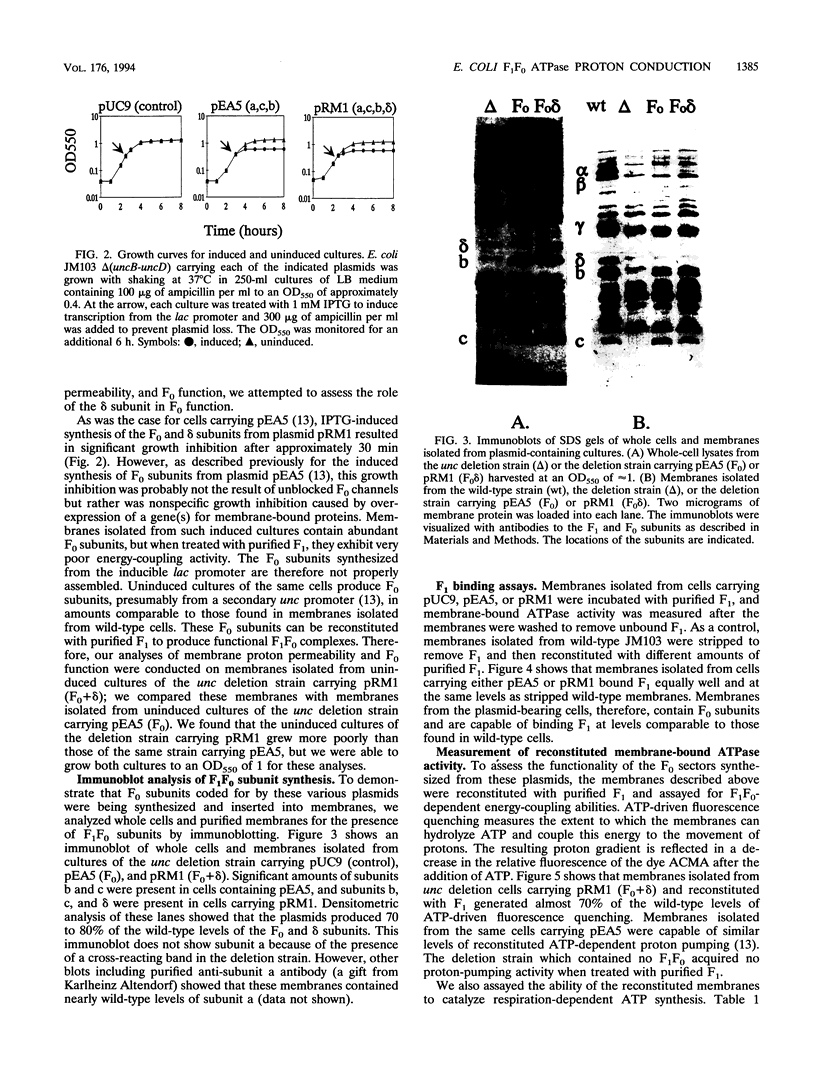
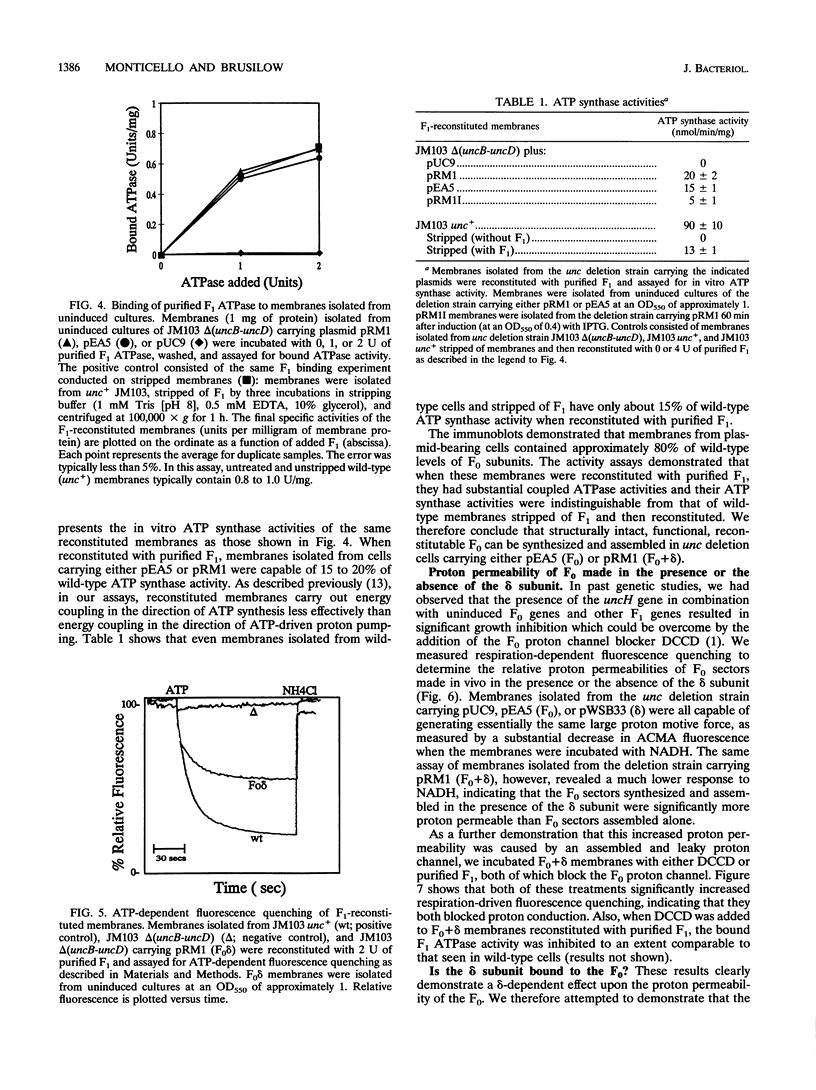
Abstract
We studied the effect of the delta subunit of the Escherichia coli F1 ATPase on the proton permeability of the F0 proton channel synthesized and assembled in vivo. Membranes isolated from an unc deletion strain carrying a plasmid containing the genes for the F0 subunits and the delta subunit were significantly more permeable to protons than membranes isolated from the same strain carrying a plasmid containing the genes for the F0 subunits alone. This increased proton permeability could be blocked by treatment with either dicyclohexyl-carbodiimide or purified F1, both of which block proton conduction through the F0. After reconstitution with purified F1 in vitro, both membrane preparations could couple proton pumping to ATP hydrolysis. These results demonstrate that an interaction between the delta subunit and the F0 during synthesis and assembly produces a significant change in the proton permeability of the F0 proton channel.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angov E., Ng T. C., Brusilow W. S. Effect of the delta subunit on assembly and proton permeability of the F0 proton channel of Escherichia coli F1F0 ATPase. J Bacteriol. 1991 Jan;173(1):407–411. doi: 10.1128/jb.173.1.407-411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow W. S. Assembly of the Escherichia coli F1F0 ATPase, a large multimeric membrane-bound enzyme. Mol Microbiol. 1993 Aug;9(3):419–424. doi: 10.1111/j.1365-2958.1993.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Brusilow W. S. Proton leakiness caused by cloned genes for the F0 sector of the proton-translocating ATPase of Escherichia coli: requirement for F1 genes. J Bacteriol. 1987 Nov;169(11):4984–4990. doi: 10.1128/jb.169.11.4984-4990.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Langman L., Senior A. E., Ash G., Fayle D. R., Gibson F. Assembly of the adenosine triphosphatase complex in Escherichia coli: assembly of F0 is dependent on the formation of specific F1 subunits. J Bacteriol. 1981 Oct;148(1):30–42. doi: 10.1128/jb.148.1.30-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht S., Junge W. Subunit delta of H(+)-ATPases: at the interface between proton flow and ATP synthesis. Biochim Biophys Acta. 1990 Feb 22;1015(3):379–390. doi: 10.1016/0005-2728(90)90072-c. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Girvin M. E., Fraga D., Zhang Y. Correlations of structure and function in H+ translocating subunit c of F1F0 ATP synthase. Ann N Y Acad Sci. 1992 Nov 30;671:323–334. doi: 10.1111/j.1749-6632.1992.tb43806.x. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Porter B., Hermolin J., White L. K. Synthesis of a functional F0 sector of the Escherichia coli H+-ATPase does not require synthesis of the alpha or beta subunits of F1. J Bacteriol. 1986 Jan;165(1):244–251. doi: 10.1128/jb.165.1.244-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Purification, reconstitution, and subunit composition. J Biol Chem. 1979 Sep 10;254(17):8230–8236. [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. Assembly of a functional F0 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1983 Aug 25;258(16):10136–10143. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticello R. A., Angov E., Brusilow W. S. Effects of inducing expression of cloned genes for the F0 proton channel of the Escherichia coli F1F0 ATPase. J Bacteriol. 1992 May;174(10):3370–3376. doi: 10.1128/jb.174.10.3370-3376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis F. J., Kanner B. I., Gutnick D. L., Postma P. W., van Dam K. Energy conservation in membranes of mutants of Escherichia coli defective in oxidative phosphorylation. Biochim Biophys Acta. 1973 Oct 19;325(1):62–71. doi: 10.1016/0005-2728(73)90151-5. [DOI] [PubMed] [Google Scholar]
- Pati S., Brusilow W. S. The roles of the alpha and gamma subunits in proton conduction through the Fo sector of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1989 Feb 15;264(5):2640–2644. [PubMed] [Google Scholar]
- Perlin D. S., Cox D. N., Senior A. E. Integration of F1 and the membrane sector of the proton-ATPase of Escherichia coli. Role of subunit "b" (uncF protein). J Biol Chem. 1983 Aug 25;258(16):9793–9800. [PubMed] [Google Scholar]
- Scarpetta M. A., Hawthorne C. A., Brusilow W. S. Characterization of semi-uncoupled hybrid Escherichia coli-Bacillus megaterium F1F0 proton-translocating ATPases. J Biol Chem. 1991 Oct 5;266(28):18567–18572. [PubMed] [Google Scholar]
- Schneider E., Altendorf K. All three subunits are required for the reconstitution of an active proton channel (F0) of Escherichia coli ATP synthase (F1F0). EMBO J. 1985 Feb;4(2):515–518. doi: 10.1002/j.1460-2075.1985.tb03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Subunit b of the membrane moiety (F0) of ATP synthase (F1F0) from Escherichia coli is indispensable for H+ translocation and binding of the water-soluble F1 moiety. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7279–7283. doi: 10.1073/pnas.81.23.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Sternweis P. C. Restoration of coupling factor activity to Escherichia coli ATPase missing the delta subunit. Biochem Biophys Res Commun. 1975 Feb 3;62(3):764–771. doi: 10.1016/0006-291x(75)90465-9. [DOI] [PubMed] [Google Scholar]
- Solomon K. A., Brusilow W. S. Effect of an uncE ribosome-binding site mutation on the synthesis and assembly of the Escherichia coli proton-translocating ATPase. J Biol Chem. 1988 Apr 15;263(11):5402–5407. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature. 1982 Aug 26;298(5877):867–869. doi: 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]