Abstract
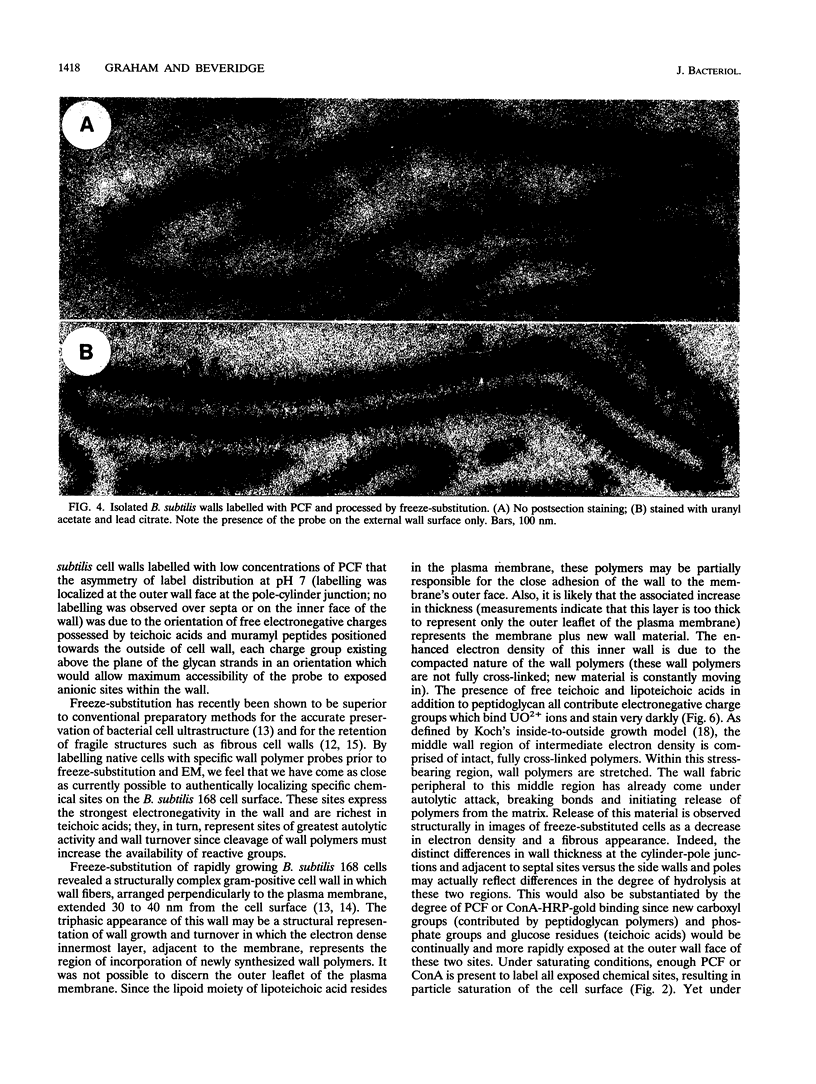
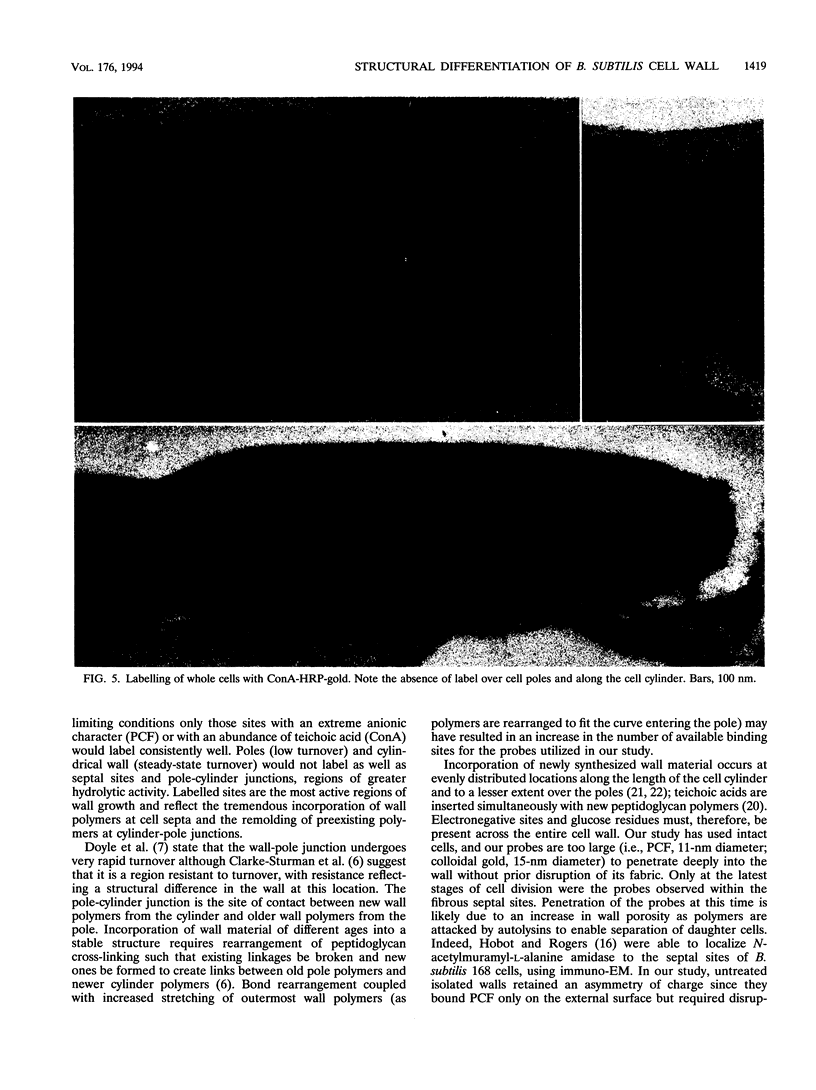
Exponential-growth-phase cultures of Bacillus subtilis 168 were probed with polycationized ferritin (PCF) or concanavalin A (localized by the addition of horseradish peroxidase conjugated to colloidal gold) to distinguish surface anionic sites and teichoic acid polymers, respectively. Isolated cell walls, lysozyme-digested cell walls, and cell walls treated with mild alkali to remove teichoic acid were also treated with PCF. After labelling, whole cells and walls were processed for electron microscopy by freeze-substitution. Thin sections of untreated cells showed a triphasic, fibrous wall extending more than 30 nm beyond the cytoplasmic membrane. Measurements of wall thickness indicated that the wall was thicker at locations adjacent to septa and at pole-cylinder junctions (P < 0.001). Labelling studies showed that at saturating concentrations the PCF probe labelled the outermost limit of the cell wall, completely surrounding individual cells. However, at limiting PCF concentrations, labelling was observed at only discrete cell surface locations adjacent to or overlying septa and at the junction between pole and cylinder. Labelling was rarely observed along the cell cylinder or directly over the poles. Cells did not label along the cylindrical wall until there was visible evidence of a developing septum. Identical labelling patterns were observed by using concanavalin A-horseradish peroxidase-colloidal gold. Neither probe appeared to penetrate between the fibers of the wall. We suggest that the fibrous appearance of the wall seen in freeze-substituted cells reflects turnover of the wall matrix, that the specificity of labelling to discrete sites on the cell surface is indicative of regions of extreme hydrolytic activity in which alpha-glucose residues of the wall teichoic acids and electronegative sites (contributed by phosphate and carboxyl groups of the teichoic acids and carboxyl groups of the peptidoglycan polymers) are more readily accessible to our probes, and that the wall of exponentially growing B. subtilis cells contains regions of structural differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Murray R. G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988 Apr;34(4):363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke-Sturman A. J., Archibald A. R., Hancock I. C., Harwood C. R., Merad T., Hobot J. A. Cell wall assembly in Bacillus subtilis: partial conservation of polar wall material and the effect of growth conditions on the pattern of incorporation of new material at the polar caps. J Gen Microbiol. 1989 Mar;135(3):657–665. doi: 10.1099/00221287-135-3-657. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Koch A. L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15(2):169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- Frehel C., Robbe P., Tinelli R., Ryter A. Relationship between biochemical and cytochemical results obtained on Bacillus megaterium and Bacillus subtilis cell-wall polysaccharides. J Ultrastruct Res. 1982 Oct;81(1):78–87. doi: 10.1016/s0022-5320(82)90042-9. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Effect of chemical fixatives on accurate preservation of Escherichia coli and Bacillus subtilis structure in cells prepared by freeze-substitution. J Bacteriol. 1990 Apr;172(4):2150–2159. doi: 10.1128/jb.172.4.2150-2159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L. Freeze-substitution studies of bacteria. Electron Microsc Rev. 1992;5(1):77–103. doi: 10.1016/0892-0354(92)90006-c. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Harris R., Villiger W., Beveridge T. J. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J Bacteriol. 1991 Mar;173(5):1623–1633. doi: 10.1128/jb.173.5.1623-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Rogers H. J. Intracellular location of the autolytic N-acetylmuramyl-L-alanine amidase in Bacillus subtilis 168 and in an autolysis-deficient mutant by immunoelectron microscopy. J Bacteriol. 1991 Feb;173(3):961–967. doi: 10.1128/jb.173.3.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J. The action of dilute alkali on some bacterial cell walls. Biochem Biophys Res Commun. 1968 Oct 10;33(1):22–28. doi: 10.1016/0006-291x(68)90248-9. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L., Williamson J. Mode of cell wall growth of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):373–378. doi: 10.1128/jb.109.1.373-378.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad T., Archibald A. R., Hancock I. C., Harwood C. R., Hobot J. A. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J Gen Microbiol. 1989 Mar;135(3):645–655. doi: 10.1099/00221287-135-3-645. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Koch A. L., Doyle R. J., Streips U. N. Insertion and fate of the cell wall in Bacillus subtilis. J Bacteriol. 1984 Apr;158(1):169–179. doi: 10.1128/jb.158.1.169-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld E. M., Beveridge T. J., Doyle R. J. Discontinuity of charge on cell wall poles of Bacillus subtilis. Can J Microbiol. 1985 Sep;31(9):875–877. doi: 10.1139/m85-163. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld E. M., Beveridge T. J., Koch A. L., Doyle R. J. Asymmetric distribution of charge on the cell wall of Bacillus subtilis. J Bacteriol. 1985 Sep;163(3):1167–1171. doi: 10.1128/jb.163.3.1167-1171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Shockman G. D., Higgins M. L. Structural arrangement of polymers within the wall of Streptococcus faecalis. J Bacteriol. 1978 Jan;133(1):372–386. doi: 10.1128/jb.133.1.372-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]