Abstract
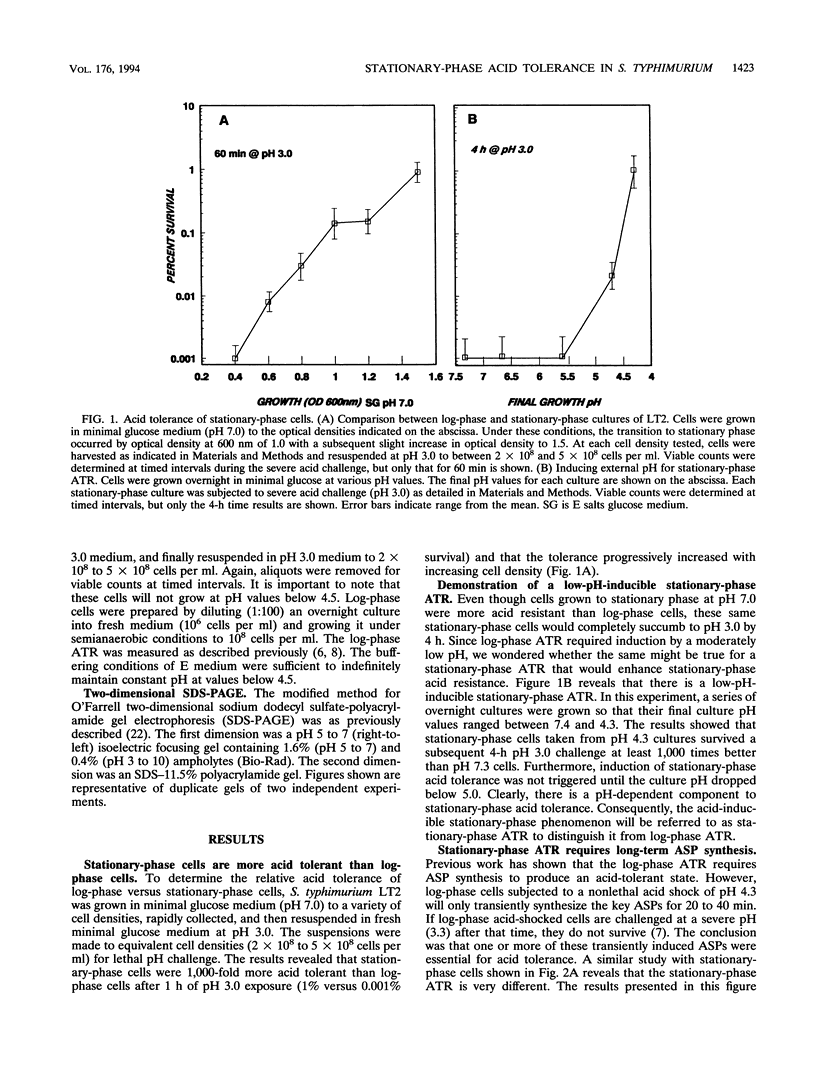
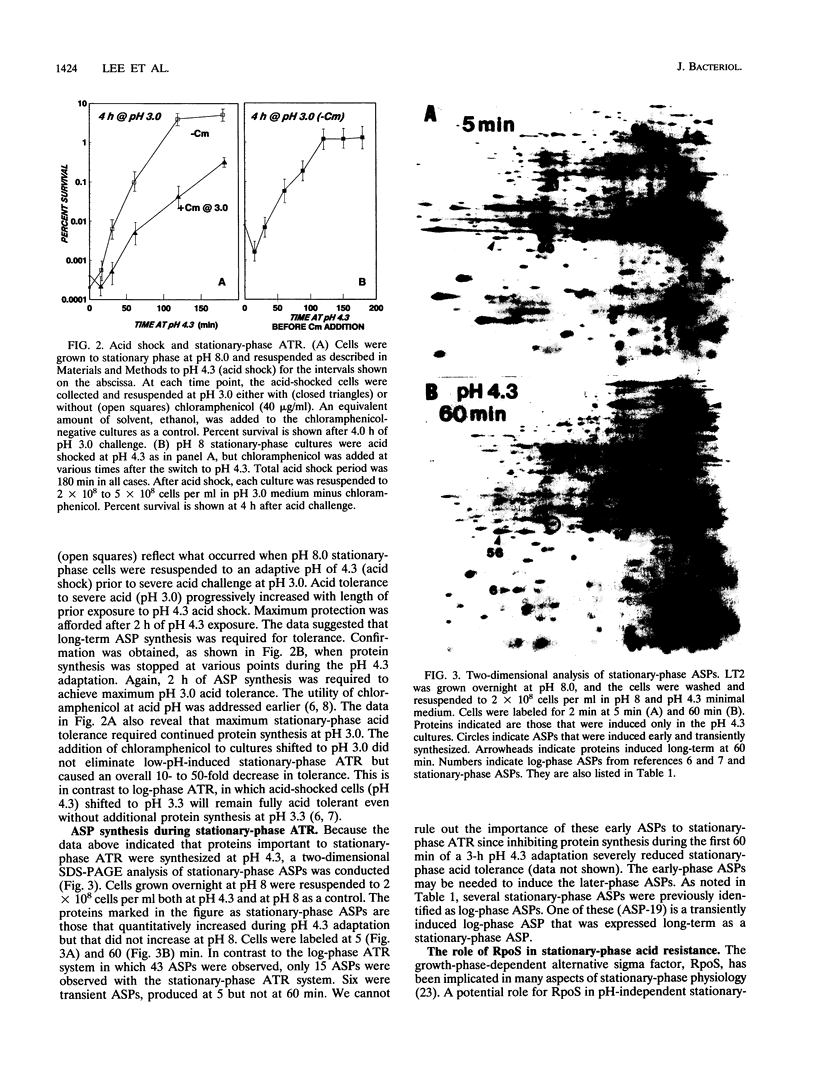
Acid is an important environmental condition encountered by Salmonella typhimurium during its pathogenesis. Our studies have shown that the organism can actively adapt to survive potentially lethal acid exposures by way of at least three possibly overlapping systems. The first is a two-stage system induced in response to low pH by logarithmic-phase cells called the log-phase acid tolerance response (ATR). It involves a major molecular realignment of the cell including the induction of over 40 proteins. The present data reveal that two additional systems of acid resistance occur in stationary-phase cells. One is a pH-dependent system distinct from log-phase ATR called stationary-phase ATR. It was shown to provide a higher level of acid resistance than log-phase ATR but involved the synthesis of fewer proteins. Maximum induction of stationary-phase ATR occurred at pH 4.3. A third system of acid resistance is not induced by low pH but appears to be part of a general stress resistance induced by stationary phase. This last system requires the alternative sigma factor, RpoS. Regulation of log-phase ATR and stationary-phase ATR remains RpoS independent. Although the three systems are for the most part distinct from each other, together they afford maximum acid resistance for S. typhimurium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abshire K. Z., Neidhardt F. C. Growth rate paradox of Salmonella typhimurium within host macrophages. J Bacteriol. 1993 Jun;175(12):3744–3748. doi: 10.1128/jb.175.12.3744-3748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli W. A., Marquis R. E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991 Apr;57(4):1134–1138. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S., Shiner M., McLeod G. M. Studies on the intestinal flora. I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology. 1969 Jan;56(1):71–79. [PubMed] [Google Scholar]
- Fang F. C., Libby S. J., Buchmeier N. A., Loewen P. C., Switala J., Harwood J., Guiney D. G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992 Jul;174(13):4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991 Nov;173(21):6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. The acid tolerance response of Salmonella typhimurium involves transient synthesis of key acid shock proteins. J Bacteriol. 1993 Apr;175(7):1981–1987. doi: 10.1128/jb.175.7.1981-1987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo F., Foster J. W., Finlay B. B. Role of acid tolerance response genes in Salmonella typhimurium virulence. Infect Immun. 1993 Oct;61(10):4489–4492. doi: 10.1128/iai.61.10.4489-4492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Broitman S. A., Zamcheck N. Influence of gastric acidity on bacterial and parasitic enteric infections. A perspective. Ann Intern Med. 1973 Feb;78(2):271–276. doi: 10.7326/0003-4819-78-2-271. [DOI] [PubMed] [Google Scholar]
- Gorden J., Small P. L. Acid resistance in enteric bacteria. Infect Immun. 1993 Jan;61(1):364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A. Relevance of inoculation route to virulence of three Salmonella spp. strains in mice. Microb Pathog. 1991 Jul;11(1):11–18. doi: 10.1016/0882-4010(91)90089-s. [DOI] [PubMed] [Google Scholar]
- Riikonen P., Mäkelä P. H., Saarilahti H., Sukupolvi S., Taira S., Rhen M. The virulence plasmid does not contribute to growth of Salmonella in cultured murine macrophages. Microb Pathog. 1992 Oct;13(4):281–291. doi: 10.1016/0882-4010(92)90038-p. [DOI] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Takayanagi Y., Fujita N., Ishihama A., Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]




