Abstract
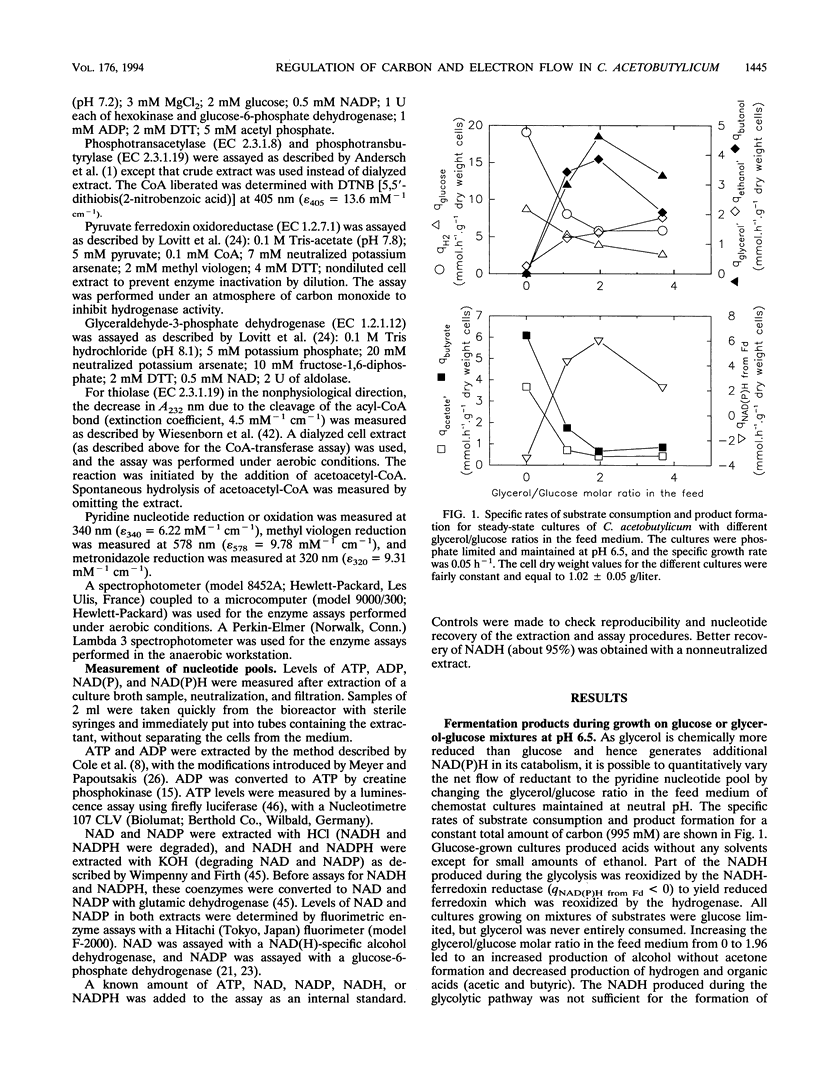
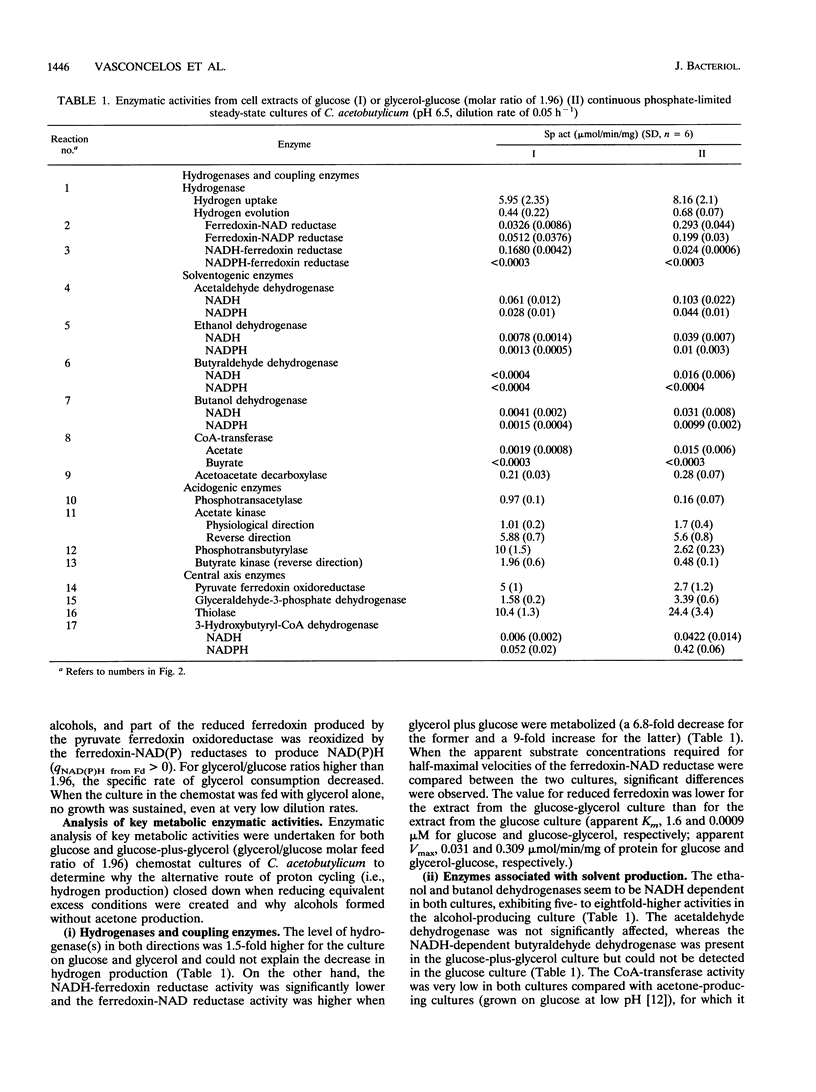
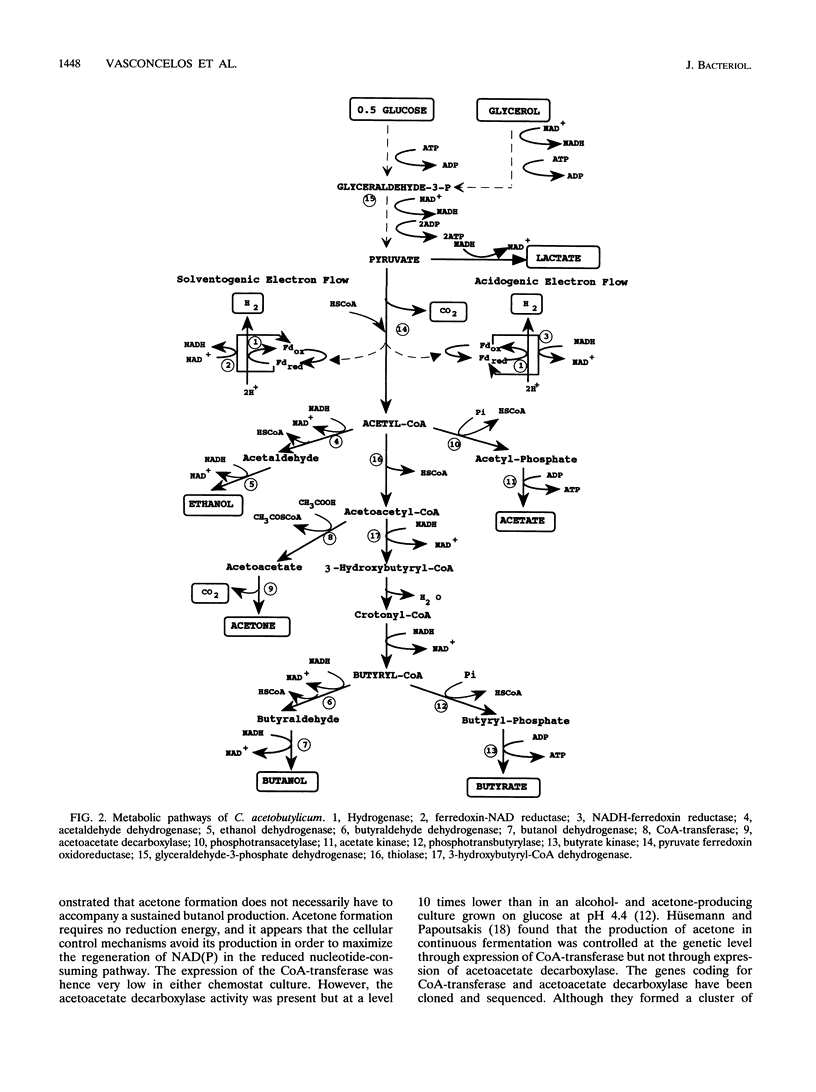
The metabolism of Clostridium acetobutylicum was manipulated, at neutral pH and in chemostat culture, by changing the overall degree of reduction of the substrate, using mixtures of glucose and glycerol. Cultures grown on glucose alone produced only acids, and the intracellular enzymatic pattern indicated the absence of butyraldehyde dehydrogenase activity and very low levels of coenzyme A-transferase, butanol, and ethanol dehydrogenase activities. In contrast, cultures grown on mixtures of glucose and glycerol produced mainly alcohols and low levels of hydrogen. The low production of hydrogen was not associated with a change in the hydrogenase level but was correlated with the induction of a ferredoxin-NAD reductase and a decreased level of NADH-ferredoxin reductase. The production of alcohols was related to the induction of a NAD-dependent butyraldehyde dehydrogenase and to higher expression of NAD-dependent ethanol and butanol dehydrogenases. The coenzyme A-transferase was poorly expressed, and thus no acetone was produced. These changes in the enzymatic pattern, obtained with cultures grown on a mixture of glucose and glycerol, were associated with a 7-fold increase of the intracellular level of NADH and a 2.5-fold increase of the level of ATP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl H., Gottwald M., Kuhn A., Rale V., Andersch W., Gottschalk G. Nutritional Factors Affecting the Ratio of Solvents Produced by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):169–172. doi: 10.1128/aem.52.1.169-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusson H., Petitdemange H., Gay R. A new, fast, and sensitive assay for NADH--ferredoxin oxidoreductase detection in clostridia. Anal Biochem. 1981 Jan 1;110(1):176–181. doi: 10.1016/0003-2697(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cary J. W., Petersen D. J., Papoutsakis E. T., Bennett G. N. Cloning and expression of Clostridium acetobutylicum phosphotransbutyrylase and butyrate kinase genes in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4613–4618. doi: 10.1128/jb.170.10.4613-4618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Datta R., Zeikus J. G. Modulation of acetone-butanol-ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol. 1985 Mar;49(3):522–529. doi: 10.1128/aem.49.3.522-529.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during the shift to solventogenesis. J Bacteriol. 1992 Jan;174(2):426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe H., Gottschalk G. Physiological Events in Clostridium acetobutylicum during the Shift from Acidogenesis to Solventogenesis in Continuous Culture and Presentation of a Model for Shift Induction. Appl Environ Microbiol. 1992 Dec;58(12):3896–3902. doi: 10.1128/aem.58.12.3896-3902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. H., Bellows P., Datta R., Zeikus J. G. Control of Carbon and Electron Flow in Clostridium acetobutylicum Fermentations: Utilization of Carbon Monoxide to Inhibit Hydrogen Production and to Enhance Butanol Yields. Appl Environ Microbiol. 1984 Oct;48(4):764–770. doi: 10.1128/aem.48.4.764-770.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol. 1980 Nov;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Knight M. Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 1966 Aug;44(2):241–254. doi: 10.1099/00221287-44-2-241. [DOI] [PubMed] [Google Scholar]
- Monot F., Martin J. R., Petitdemange H., Gay R. Acetone and Butanol Production by Clostridium acetobutylicum in a Synthetic Medium. Appl Environ Microbiol. 1982 Dec;44(6):1318–1324. doi: 10.1128/aem.44.6.1318-1324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum. Methods Enzymol. 1978;53:286–296. doi: 10.1016/s0076-6879(78)53035-8. [DOI] [PubMed] [Google Scholar]
- Petersen D. J., Cary J. W., Vanderleyden J., Bennett G. N. Sequence and arrangement of genes encoding enzymes of the acetone-production pathway of Clostridium acetobutylicum ATCC824. Gene. 1993 Jan 15;123(1):93–97. doi: 10.1016/0378-1119(93)90545-e. [DOI] [PubMed] [Google Scholar]
- Petersen D. J., Welch R. W., Rudolph F. B., Bennett G. N. Molecular cloning of an alcohol (butanol) dehydrogenase gene cluster from Clostridium acetobutylicum ATCC 824. J Bacteriol. 1991 Mar;173(5):1831–1834. doi: 10.1128/jb.173.5.1831-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Cherrier C., Raval R., Gay R. Regulation of the NADH and NADPH-ferredoxin oxidoreductases in clostridia of the butyric group. Biochim Biophys Acta. 1976 Feb 24;421(2):334–337. doi: 10.1016/0304-4165(76)90300-7. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl R. C., Orme-Johnson W. H. Clostridial pyruvate oxidoreductase and the pyruvate-oxidizing enzyme specific to nitrogen fixation in Klebsiella pneumoniae are similar enzymes. J Biol Chem. 1987 Aug 5;262(22):10489–10496. [PubMed] [Google Scholar]
- Walter K. A., Bennett G. N., Papoutsakis E. T. Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol. 1992 Nov;174(22):7149–7158. doi: 10.1128/jb.174.22.7149-7158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. W., Rudolph F. B., Papoutsakis E. T. Purification and characterization of the NADH-dependent butanol dehydrogenase from Clostridium acetobutylicum (ATCC 824). Arch Biochem Biophys. 1989 Sep;273(2):309–318. doi: 10.1016/0003-9861(89)90489-x. [DOI] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989 Feb;55(2):323–329. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl Environ Microbiol. 1989 Feb;55(2):317–322. doi: 10.1128/aem.55.2.317-322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Thiolase from Clostridium acetobutylicum ATCC 824 and Its Role in the Synthesis of Acids and Solvents. Appl Environ Microbiol. 1988 Nov;54(11):2717–2722. doi: 10.1128/aem.54.11.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


