Abstract
A genetic approach was used to study (dissimilatory) ferric iron (Fe3+) reduction in Shewanella putrefaciens 200. Chemical mutagenesis procedures and two rapid plate assays were developed to facilitate the screening of Fe3+ reduction-deficient mutants. Sixty-two putative Fe3+ reduction-deficient mutants were identified, and each was subsequently tested for its ability to grow anaerobically on various compounds as sole terminal electron acceptors, including Fe3+, nitrate (NO3-), nitrite (NO2-), manganese oxide (Mn4+), sulfite (SO3(2-)), thiosulfate (S2O3(2-)), trimethylamine N-oxide, and fumarate. A broad spectrum of mutants deficient in anaerobic growth on one or more electron acceptors was identified. Nine of the 62 mutants (designated Fer mutants) were deficient only in anaerobic growth on Fe3+ and retained the ability to grow on all other electron acceptors. These results suggest that S. putrefaciens expresses at least one terminal Fe3+ reductase that is distinct from other terminal reductases coupled to anaerobic growth. The nine Fer mutants were conjugally mated with an S. putrefaciens genomic library harbored in Escherichia coli S17-1. Complemented S. putrefaciens transconjugants were identified by the acquired ability to grow anaerobically on Fe3+ as the sole terminal electron acceptor. All recombinant cosmids that conferred the Fer+ phenotype appeared to carry a common internal region.
Full text
PDF

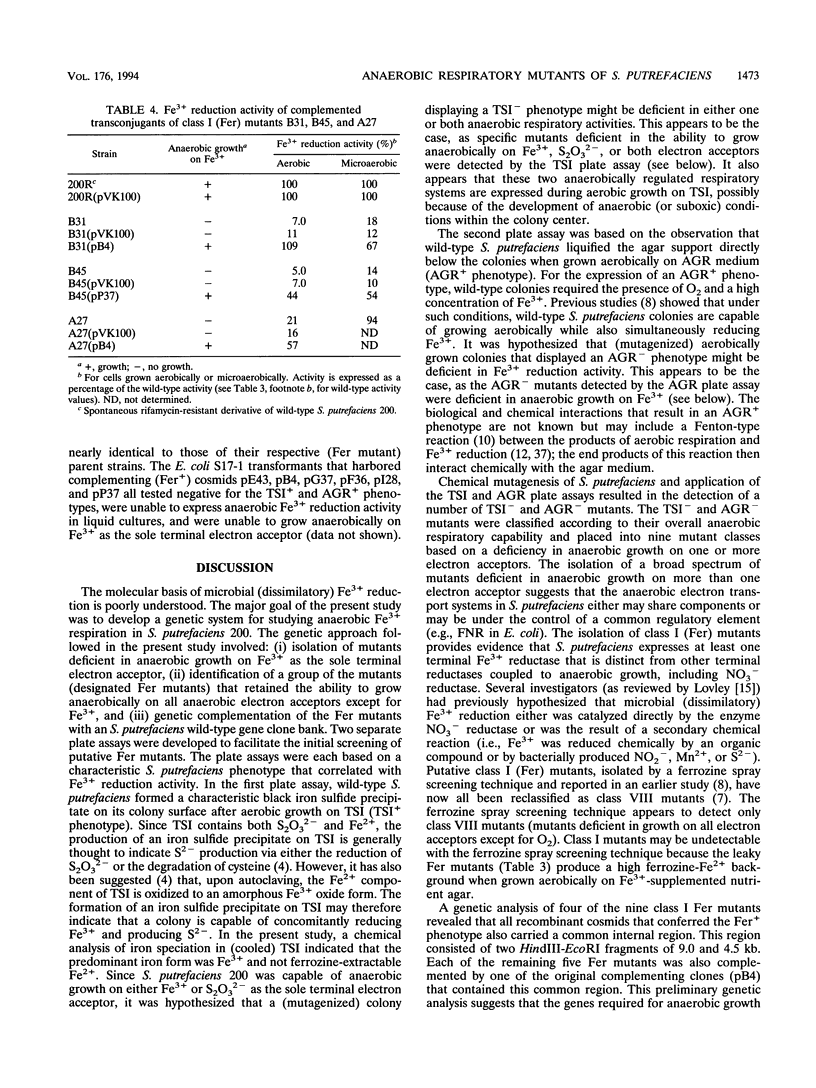

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. G., DiChristina T. J., Hoffmann M. R. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 ("Pseudomonas ferrireductans") Appl Environ Microbiol. 1986 Aug;52(2):281–289. doi: 10.1128/aem.52.2.281-289.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. G., Hoffmann M. R., Dichristina T. J., Picardal F. W. Regulation of Dissimilatory Fe(III) Reduction Activity in Shewanella putrefaciens. Appl Environ Microbiol. 1990 Sep;56(9):2811–2817. doi: 10.1128/aem.56.9.2811-2817.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Clark M. A. Tetrathionate reduction and production of hydrogen sulfide from thiosulfate. Microbiol Rev. 1987 Jun;51(2):192–205. doi: 10.1128/mr.51.2.192-205.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdige D. J., Nealson K. H. Microbial manganese reduction by enrichment cultures from coastal marine sediments. Appl Environ Microbiol. 1985 Aug;50(2):491–497. doi: 10.1128/aem.50.2.491-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChristina T. J., DeLong E. F. Design and application of rRNA-targeted oligonucleotide probes for the dissimilatory iron- and manganese-reducing bacterium Shewanella putrefaciens. Appl Environ Microbiol. 1993 Dec;59(12):4152–4160. doi: 10.1128/aem.59.12.4152-4160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChristina T. J. Effects of nitrate and nitrite on dissimilatory iron reduction by Shewanella putrefaciens 200. J Bacteriol. 1992 Mar;174(6):1891–1896. doi: 10.1128/jb.174.6.1891-1896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby Y. A., Lovley D. R. Electron Transport in the Dissimilatory Iron Reducer, GS-15. Appl Environ Microbiol. 1991 Mar;57(3):867–870. doi: 10.1128/aem.57.3.867-870.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991 Jun;55(2):259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Giovannoni S. J., White D. C., Champine J. E., Phillips E. J., Gorby Y. A., Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159(4):336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J., Lonergan D. J. Hydrogen and Formate Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989 Mar;55(3):700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J Bacteriol. 1990 Nov;172(11):6232–6238. doi: 10.1128/jb.172.11.6232-6238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W., Cook F. D. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can J Microbiol. 1981 Jul;27(7):692–697. doi: 10.1139/m81-107. [DOI] [PubMed] [Google Scholar]
- Obuekwe C. O., Westlake D. W. Effects of medium composition on cell pigmentation, cytochrome content, and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can J Microbiol. 1982 Aug;28(8):989–992. doi: 10.1139/m82-148. [DOI] [PubMed] [Google Scholar]
- Roden E. E., Lovley D. R. Dissimilatory Fe(III) Reduction by the Marine Microorganism Desulfuromonas acetoxidans. Appl Environ Microbiol. 1993 Mar;59(3):734–742. doi: 10.1128/aem.59.3.734-742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachon P. Ferric and cupric ions requirement for DNA single-strand breakage by H2O2. Free Radic Res Commun. 1989;7(1):1–10. doi: 10.3109/10715768909088155. [DOI] [PubMed] [Google Scholar]