Abstract
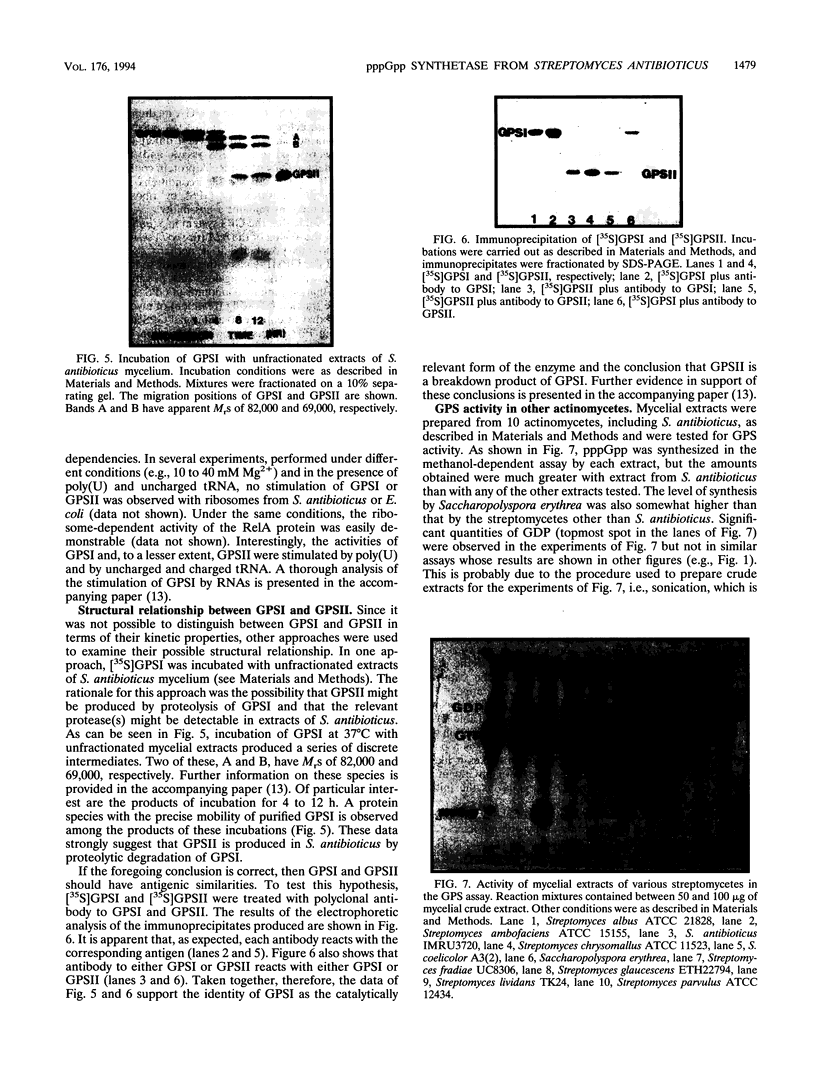
Two forms of ATP:GTP 3'-pyrophosphotransferase (guanosine pentaphosphate synthetase) have been purified from Streptomyces antibioticus. The larger form has an M(r) of 88,000, while the M(r) of a smaller form is 47,000. Both synthetase forms are active in the formation of guanosine 5'-triphosphate, 3'-diphosphate in reaction mixtures containing methanol. Unlike the RelA protein from Escherichia coli, the synthetases from S. antibioticus do not use GDP efficiently as a substrate. Experiments using crude extracts of S. antibioticus mycelium and the 88,000-M(r) form of guanosine pentaphosphate synthetase strongly suggest that the 47,000-M(r) species is produced by proteolysis of the larger species. This conclusion is supported by the observation that antibody to either protein reacts with the other protein. Thus, the 88,000-M(r) species may be the catalytically relevant protein in vivo. Unlike the RelA protein, the 88,000-M(r) protein is not activated by ribosomes. Modest levels of guanosine pentaphosphate synthesis were observed in mycelial extracts derived from nine other actinomycetes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Choy H. A., Jones G. H. Phenoxazinone synthase from Streptomyces antibiotics: purification of the large and small enzyme forms. Arch Biochem Biophys. 1981 Oct 1;211(1):55–65. doi: 10.1016/0003-9861(81)90429-x. [DOI] [PubMed] [Google Scholar]
- Fehr S., Richter D. Stringent response of Bacillus stearothermophilus: evidence for the existence of two distinct guanosine 3',5'-polyphosphate synthetases. J Bacteriol. 1981 Jan;145(1):68–73. doi: 10.1128/jb.145.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A., Sy J. Guanosine 5'-triphosphate, 3'-diphosphate 5'-phosphohydrolase. Purification and substrate specificity. J Biol Chem. 1983 Feb 10;258(3):1678–1683. [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez V. J., Bremer H. Escherichia coli ppGpp synthetase II activity requires spoT. J Biol Chem. 1991 Mar 25;266(9):5991–5999. [PubMed] [Google Scholar]
- Jones G. H. Activation of ATP:GTP 3'-pyrophosphotransferase (guanosine pentaphosphate synthetase) in Streptomyces antibioticus. J Bacteriol. 1994 Mar;176(5):1482–1487. doi: 10.1128/jb.176.5.1482-1487.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H. Combined purification of actinomycin synthetase I and 3-hydroxyanthranilic acid 4-methyltransferase from Streptomyces antibioticus. J Biol Chem. 1993 Apr 5;268(10):6831–6834. [PubMed] [Google Scholar]
- Jones G. H. Macromolecular synthesis in Streptomyces antibioticus: in vitro systems for aminoacylation and translation from young and old cells. J Bacteriol. 1975 Oct;124(1):364–372. doi: 10.1128/jb.124.1.364-372.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Kelly K. S., Ochi K., Jones G. H. Pleiotropic effects of a relC mutation in Streptomyces antibioticus. J Bacteriol. 1991 Apr;173(7):2297–2300. doi: 10.1128/jb.173.7.2297-2300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metzger S., Dror I. B., Aizenman E., Schreiber G., Toone M., Friesen J. D., Cashel M., Glaser G. The nucleotide sequence and characterization of the relA gene of Escherichia coli. J Biol Chem. 1988 Oct 25;263(30):15699–15704. [PubMed] [Google Scholar]
- Mukai J., Muta S., Ozumi Y. Streptomyces nucleotide 3'-pyrophosphokinase are insensitive to stringent control. J Basic Microbiol. 1989;29(10):671–674. doi: 10.1002/jobm.3620291008. [DOI] [PubMed] [Google Scholar]
- Ochi K. Streptomyces relC mutants with an altered ribosomal protein ST-L11 and genetic analysis of a Streptomyces griseus relC mutant. J Bacteriol. 1990 Jul;172(7):4008–4016. doi: 10.1128/jb.172.7.4008-4016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Kjeldgaard N. O. Analysis of the relA gene product of Escherichia coli. Eur J Biochem. 1977 Jun 1;76(1):91–97. doi: 10.1111/j.1432-1033.1977.tb11573.x. [DOI] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Reiness G., Yang H. L., Zubay G., Cashel M. Effects of guanosine tetraphosphate on cell-free synthesis of Escherichia coli ribosomal RNA and other gene products. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2881–2885. doi: 10.1073/pnas.72.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D., Fehr S., Harder R. The guanosine 3',5'-bis(diphosphate) (ppGpp) cycle. Comparison of synthesis and degradation of guanosine 3',5'-bis(diphosphate) in various bacterial systems. Eur J Biochem. 1979 Aug 15;99(1):57–64. doi: 10.1111/j.1432-1033.1979.tb13230.x. [DOI] [PubMed] [Google Scholar]
- Riggs D. L., Mueller R. D., Kwan H. S., Artz S. W. Promoter domain mediates guanosine tetraphosphate activation of the histidine operon. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9333–9337. doi: 10.1073/pnas.83.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P., Thang M. N. Membrane associated proteases in E. coli. FEBS Lett. 1973 Oct 1;36(1):31–33. doi: 10.1016/0014-5793(73)80330-8. [DOI] [PubMed] [Google Scholar]
- Sarubbi E., Rudd K. E., Xiao H., Ikehara K., Kalman M., Cashel M. Characterization of the spoT gene of Escherichia coli. J Biol Chem. 1989 Sep 5;264(25):15074–15082. [PubMed] [Google Scholar]
- Schreiber G., Metzger S., Aizenman E., Roza S., Cashel M., Glaser G. Overexpression of the relA gene in Escherichia coli. J Biol Chem. 1991 Feb 25;266(6):3760–3767. [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Strauch E., Takano E., Baylis H. A., Bibb M. J. The stringent response in Streptomyces coelicolor A3(2). Mol Microbiol. 1991 Feb;5(2):289–298. doi: 10.1111/j.1365-2958.1991.tb02109.x. [DOI] [PubMed] [Google Scholar]
- Sy J. A ribosome-independent, soluble stringent factor-like enzyme isolated from a Bacillus brevis. Biochemistry. 1976 Feb 10;15(3):606–609. doi: 10.1021/bi00648a024. [DOI] [PubMed] [Google Scholar]
- Takano E., Gramajo H. C., Strauch E., Andres N., White J., Bibb M. J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol Microbiol. 1992 Oct;6(19):2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991 Mar 25;266(9):5980–5990. [PubMed] [Google Scholar]








