Abstract
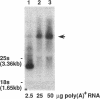
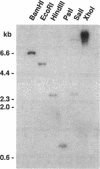
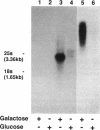
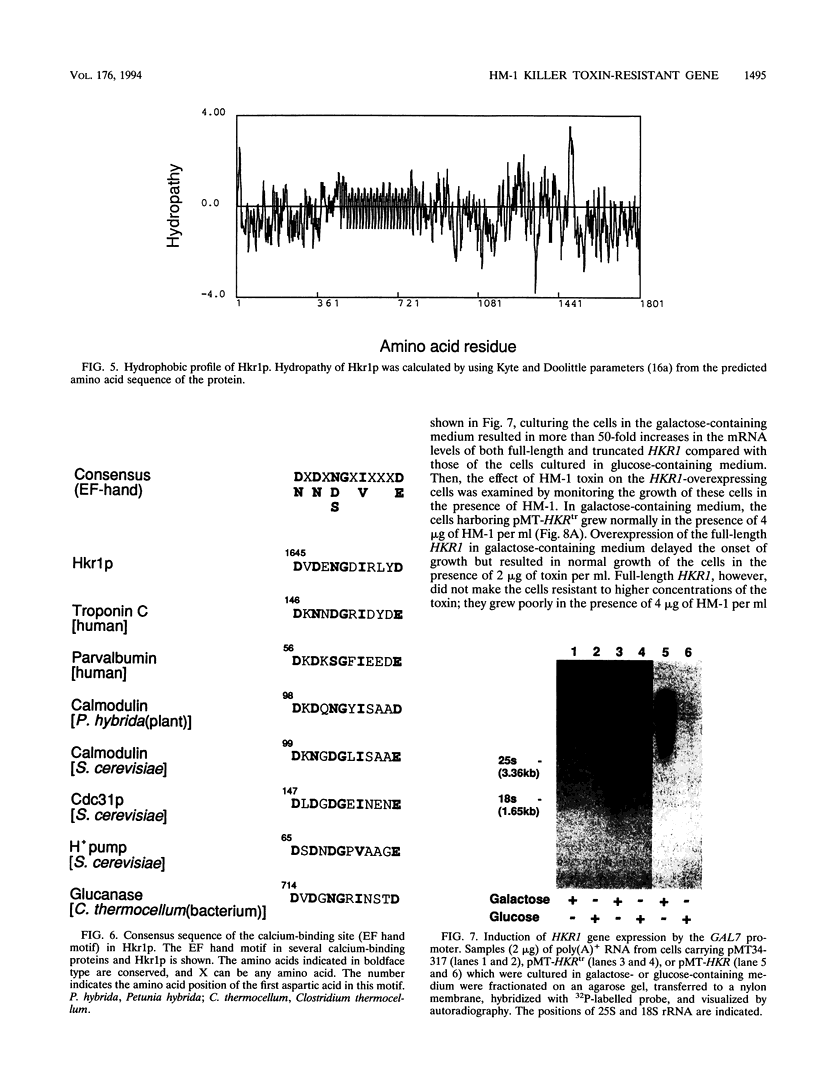
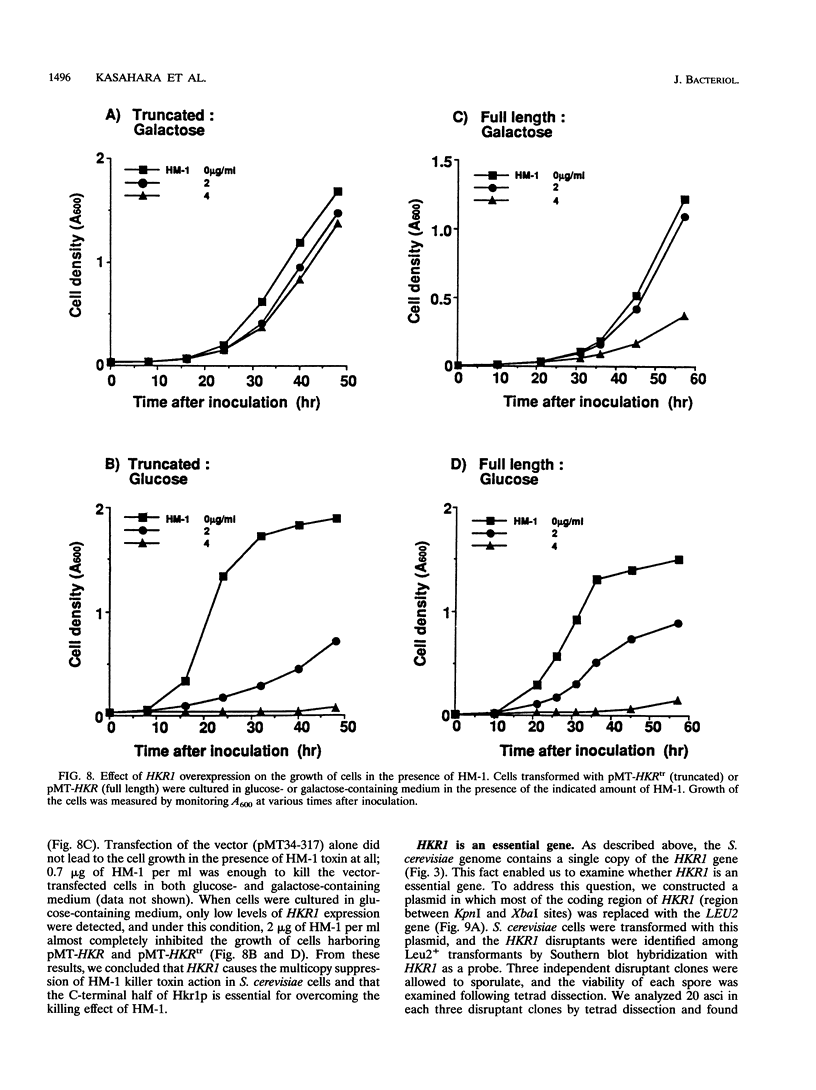
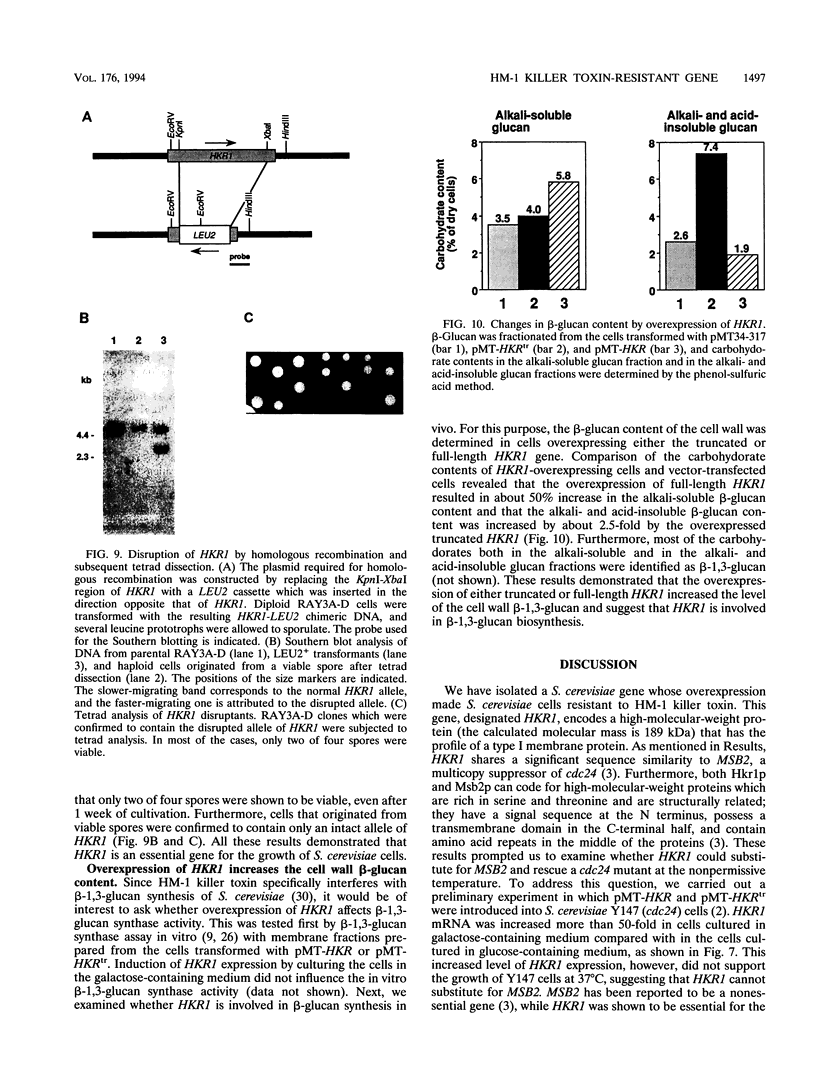
A gene whose overexpression can endow Saccharomyces cerevisiae cells with resistance to HM-1 killer toxin was cloned from an S. cerevisiae genomic library. This gene, designated HKR1 (Hansenula mrakii killer toxin-resistant gene 1), contains a 5.4-kb open reading frame. The predicted amino acid sequence of the protein specified by HKR1 indicates that the protein consists of 1,802 amino acids and is very rich in serine and threonine, which could serve as O-glycosylation sites. The protein also contains two hydrophobic domains at the N-terminal end and in the C-terminal half, which could function as a signal peptide and transmembrane domain, respectively. Hkr1p is found to contain an EF hand motif of the calcium-binding consensus sequence in the C-terminal cytoplasmic domain. Thus, Hkr1p is expected to be a calcium-binding, glycosylated type I membrane protein. Southern and Northern (RNA) analyses demonstrated that there is a single copy of the HKR1 gene in the S. cerevisiae genome, and the transcriptional level of HKR1 is extremely low. Gene disruption followed by tetrad analysis showed that HKR1 is an essential gene. Overexpression of the truncated HKR1 encoding the C-terminal half of Hkr1p made the cells more resistant to HM-1 killer toxin than the full-length HKR1 did, demonstrating that the C-terminal half of Hkr1p is essential for overcoming the effect of HM-1 killer toxin. Furthermore, overexpression of HKR1 increased the beta-glucan content in the cell wall without affecting in vitro beta-glucan synthase activity, suggesting that HKR1 regulates beta-glucan synthesis in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Bender A., Pringle J. R. A Ser/Thr-rich multicopy suppressor of a cdc24 bud emergence defect. Yeast. 1992 Apr;8(4):315–323. doi: 10.1002/yea.320080409. [DOI] [PubMed] [Google Scholar]
- Bender A., Pringle J. R. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C., Sommer S. S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J Cell Biol. 1990 May;110(5):1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Bussey H. The yeast KRE9 gene encodes an O glycoprotein involved in cell surface beta-glucan assembly. Mol Cell Biol. 1993 Oct;13(10):6346–6356. doi: 10.1128/mcb.13.10.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Kossaczka Z., Jiang B., Bussey H. A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1-->6)-beta-glucan synthesis. Genetics. 1993 Apr;133(4):837–849. doi: 10.1093/genetics/133.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Saville D., Hutchins K., Palfree R. G. Binding of yeast killer toxin to a cell wall receptor on sensitive Saccharomyces cerevisiae. J Bacteriol. 1979 Dec;140(3):888–892. doi: 10.1128/jb.140.3.888-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Kang M. S. Fungal 1,3-beta-glucan synthase. Methods Enzymol. 1987;138:637–642. doi: 10.1016/0076-6879(87)38057-7. [DOI] [PubMed] [Google Scholar]
- Hill K., Boone C., Goebl M., Puccia R., Sdicu A. M., Bussey H. Yeast KRE2 defines a new gene family encoding probable secretory proteins, and is required for the correct N-glycosylation of proteins. Genetics. 1992 Feb;130(2):273–283. doi: 10.1093/genetics/130.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins K., Bussey H. Cell wall receptor for yeast killer toxin: involvement of (1 leads to 6)-beta-D-glucan. J Bacteriol. 1983 Apr;154(1):161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. S., Cabib E. Regulation of fungal cell wall growth: a guanine nucleotide-binding, proteinaceous component required for activity of (1----3)-beta-D-glucan synthase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5808–5812. doi: 10.1073/pnas.83.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Kuribayashi-Ohta K., Tamatsukuri S., Hikata M., Miyamoto C., Furuichi Y. Application of oligo(dT)30-latex for rapid purification of poly(A)+ mRNA and for hybrid subtraction with the in situ reverse transcribed cDNA. Biochim Biophys Acta. 1993 Feb 13;1156(2):204–212. doi: 10.1016/0304-4165(93)90137-w. [DOI] [PubMed] [Google Scholar]
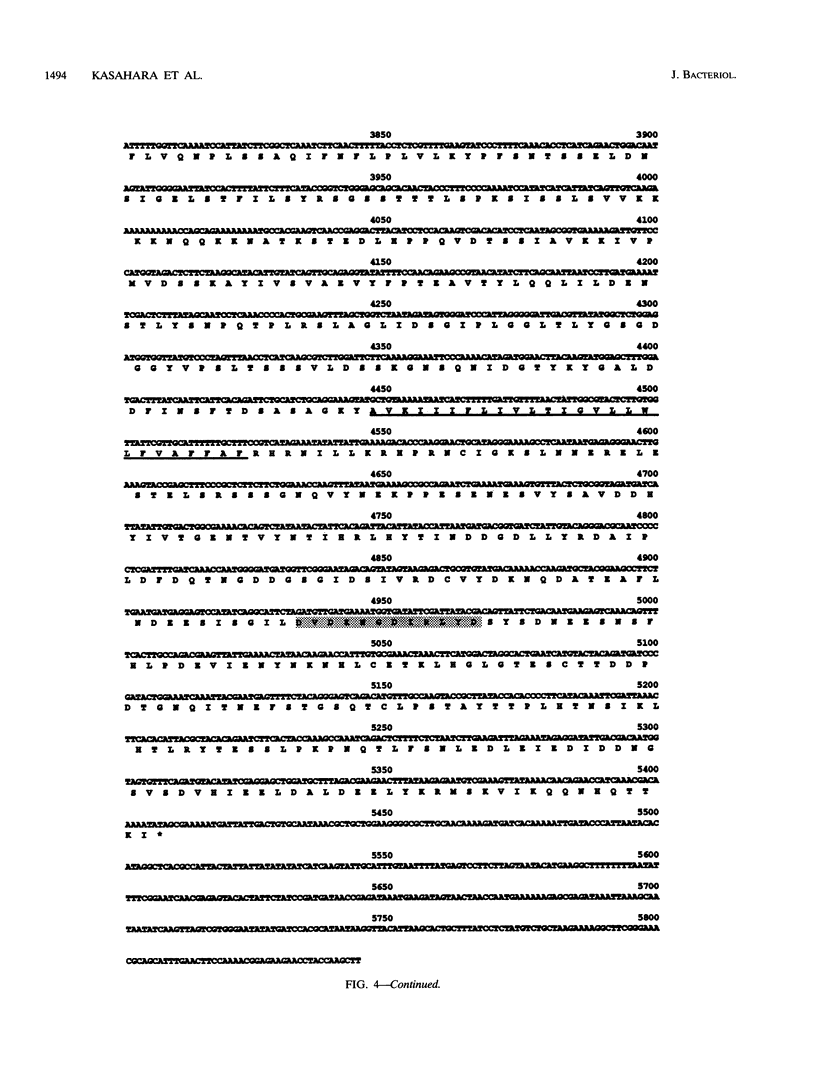
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaden P., Hill K., Wagner J., Slipetz D., Sommer S. S., Bussey H. The yeast KRE5 gene encodes a probable endoplasmic reticulum protein required for (1----6)-beta-D-glucan synthesis and normal cell growth. Mol Cell Biol. 1990 Jun;10(6):3013–3019. doi: 10.1128/mcb.10.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notario V., Kawai H., Cabib E. Interaction between yeast beta-(1 goes to 3)glucan synthetase and activating phosphorylated compounds. A kinetic study. J Biol Chem. 1982 Feb 25;257(4):1902–1905. [PubMed] [Google Scholar]
- Roemer T., Bussey H. Yeast beta-glucan synthesis: KRE6 encodes a predicted type II membrane protein required for glucan synthesis in vivo and for glucan synthase activity in vitro. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11295–11299. doi: 10.1073/pnas.88.24.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Shematek E. M., Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of beta-(1 leads to 3)glucan synthetase by ATP and GTP. J Biol Chem. 1980 Feb 10;255(3):895–902. [PubMed] [Google Scholar]
- Tajima M., Nogi Y., Fukasawa T. Duplicate upstream activating sequences in the promoter region of the Saccharomyces cerevisiae GAL7 gene. Mol Cell Biol. 1986 Jan;6(1):246–256. doi: 10.1128/mcb.6.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Hiratani T., Hirata H., Imai M., Yamaguchi H. Killer toxin from Hansenula mrakii selectively inhibits cell wall synthesis in a sensitive yeast. FEBS Lett. 1986 Mar 3;197(1-2):50–54. doi: 10.1016/0014-5793(86)80296-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Imai M., Tachibana K., Mayumi M. Application of monoclonal antibodies to the isolation and characterization of a killer toxin secreted by Hansenula mrakii. FEBS Lett. 1986 Jan 20;195(1-2):253–257. doi: 10.1016/0014-5793(86)80170-3. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]