Abstract
Bradyrhizobium japonicum produces delta-aminolevulinic acid, the universal precursor of tetrapyrroles, in a reaction catalyzed by the product of the hemA gene. Expression of the B. japonicum hemA gene is affected by iron availability. Activity of a hemA-lacZ fusion is increased approximately threefold by iron, and RNA analysis indicates that iron regulation is at the level of mRNA accumulation. To our knowledge, this is the first example of an iron-regulated heme biosynthetic gene in prokaryotes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., McClung C. R., Chelm B. K. Physical organization of the Bradyrhizobium japonicum nitrogenase gene region. J Bacteriol. 1984 Sep;159(3):857–862. doi: 10.1128/jb.159.3.857-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Ormerod J. G., Beale S. I. Distribution of delta-aminolevulinic acid biosynthetic pathways among phototrophic bacterial groups. Arch Microbiol. 1989;151(6):513–519. doi: 10.1007/BF00454867. [DOI] [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovy A., de Vrieze G., Lugones L., van Horssen P., van den Berg C., Borrias M., Weisbeek P. Iron-dependent stability of the ferredoxin I transcripts from the cyanobacterial strains Synechococcus species PCC 7942 and Anabaena species PCC 7937. Mol Microbiol. 1993 Feb;7(3):429–439. doi: 10.1111/j.1365-2958.1993.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M. J., Storey D. G. Zinc and iron regulate translation of the gene encoding Pseudomonas aeruginosa elastase. Mol Microbiol. 1992 Feb;6(3):337–344. doi: 10.1111/j.1365-2958.1992.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- David M., Daveran M. L., Batut J., Dedieu A., Domergue O., Ghai J., Hertig C., Boistard P., Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988 Aug 26;54(5):671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
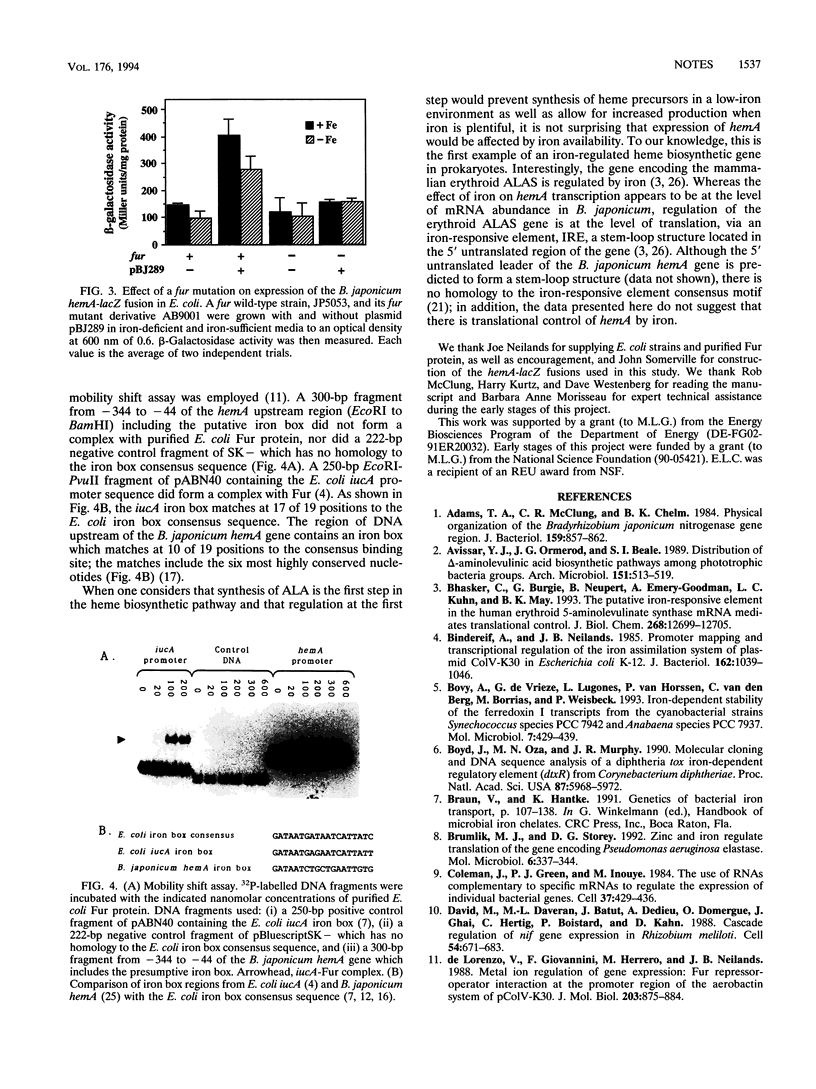
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Ditta G. S., Helinski D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991 Mar 14;350(6314):170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Bacterial delta-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Meidl E. J., Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990 Jun;172(6):3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Litwin C. M., Boyko S. A., Calderwood S. B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992 Mar;174(6):1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Calderwood S. B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993 Feb;175(3):706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Calderwood S. B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993 Apr;6(2):137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C. R., Somerville J. E., Guerinot M. L., Chelm B. K. Structure of the Bradyrhizobium japonicum gene hemA encoding 5-aminolevulinic acid synthase. Gene. 1987;54(1):133–139. doi: 10.1016/0378-1119(87)90355-6. [DOI] [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Preisig O., Anthamatten D., Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. W., Cox C. D., Vasil M. L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993 May;175(9):2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler P. G., Nadler K. D. Effects of iron deficiency on heme biosynthesis in Rhizobium japonicum. J Bacteriol. 1982 Mar;149(3):1021–1026. doi: 10.1128/jb.149.3.1021-1026.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Craig J. S., Guerinot M. L., Chelm B. K. Isolation of Bradyrhizobium japonicum DNA sequences that are transcribed at high levels in bacteroids. Mol Gen Genet. 1991 Sep;228(3):356–360. doi: 10.1007/BF00260627. [DOI] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Fur regulation in Yersinia species. Mol Microbiol. 1992 Sep;6(17):2507–2516. doi: 10.1111/j.1365-2958.1992.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Tao X., Boyd J., Murphy J. R. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5897–5901. doi: 10.1073/pnas.89.13.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Murphy J. R. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires a divalent heavy metal ion and protects the palindromic sequence from DNase I digestion. J Biol Chem. 1992 Oct 25;267(30):21761–21764. [PubMed] [Google Scholar]
- Waldbeser L. S., Tolmasky M. E., Actis L. A., Crosa J. H. Mechanisms for negative regulation by iron of the fatA outer membrane protein gene expression in Vibrio anguillarum 775. J Biol Chem. 1993 May 15;268(14):10433–10439. [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]