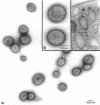
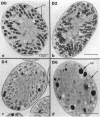
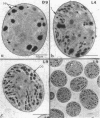
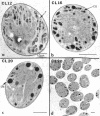
Abstract
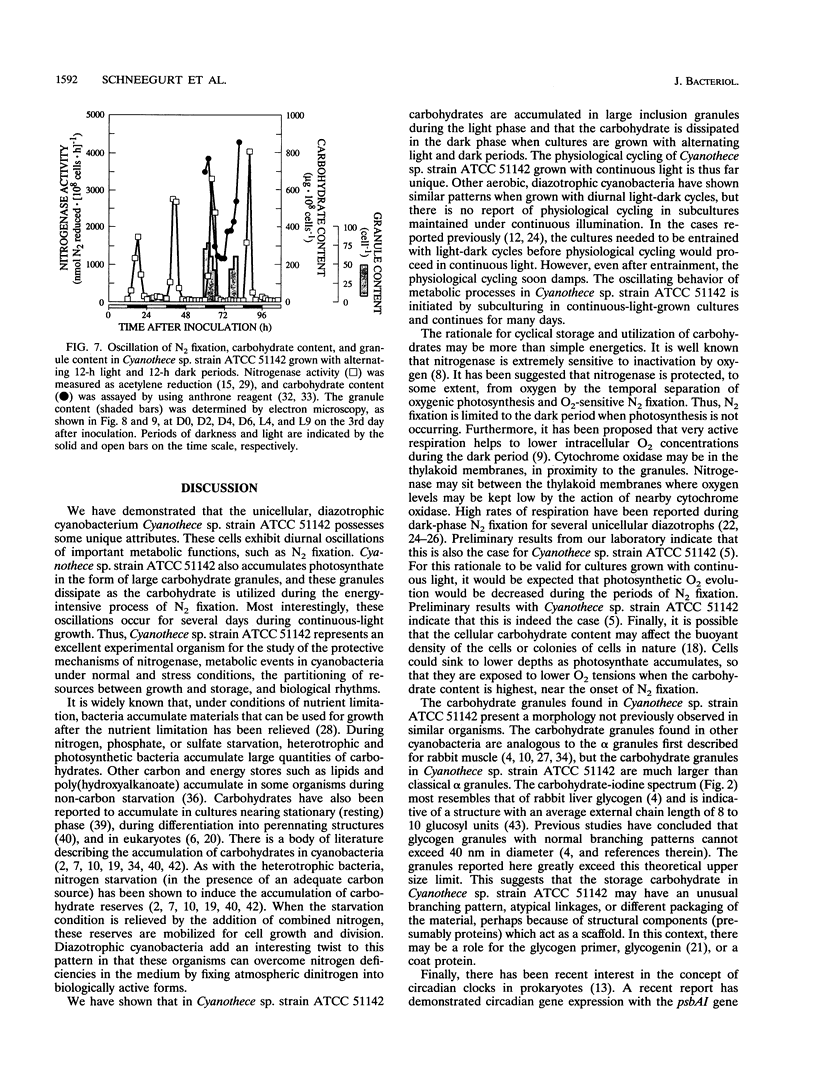
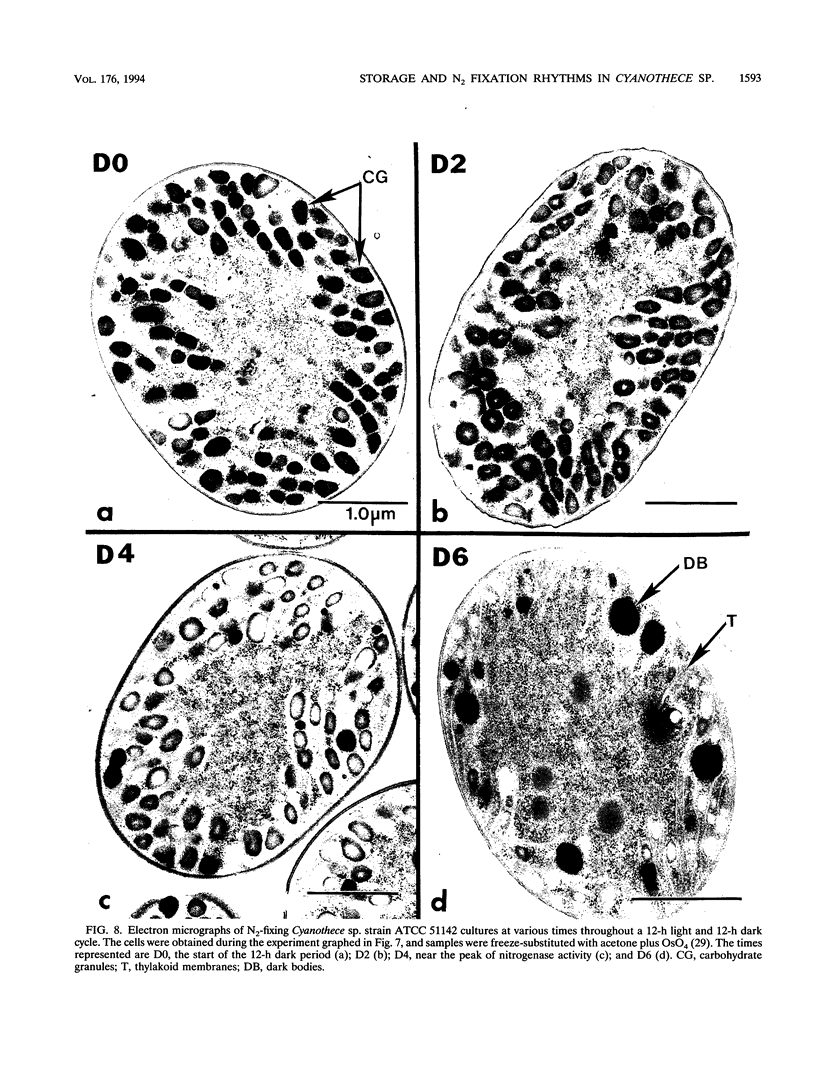
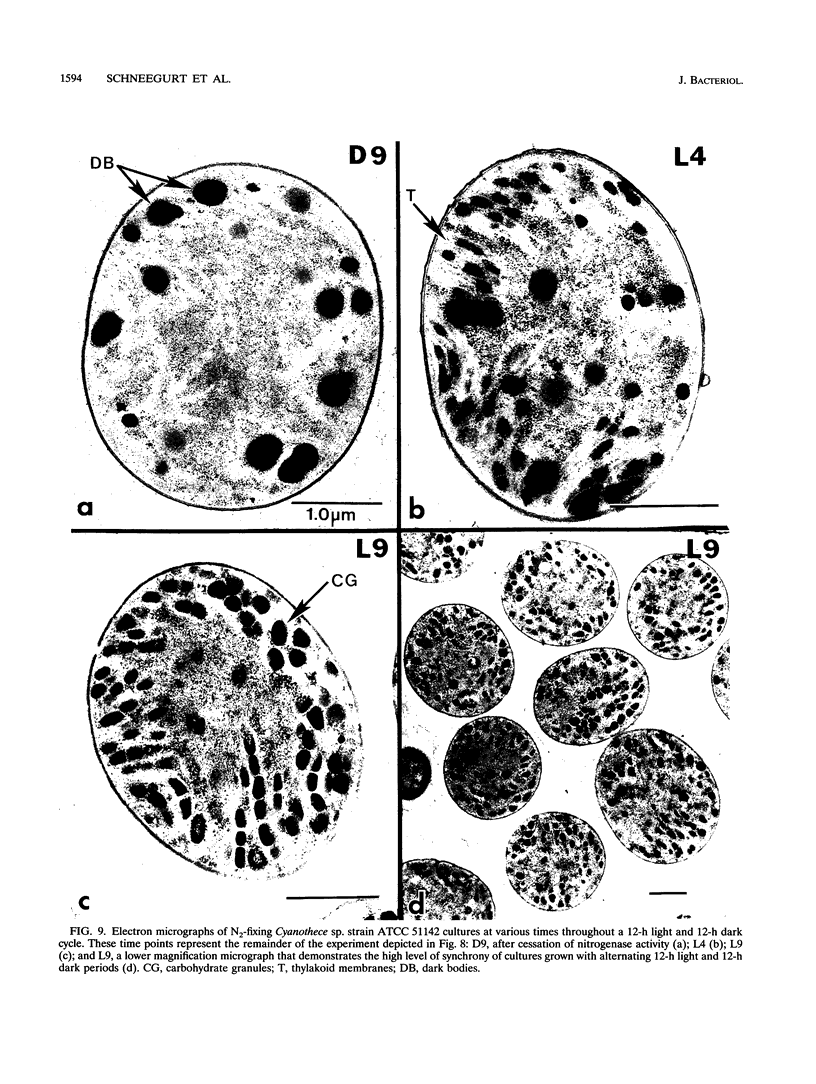
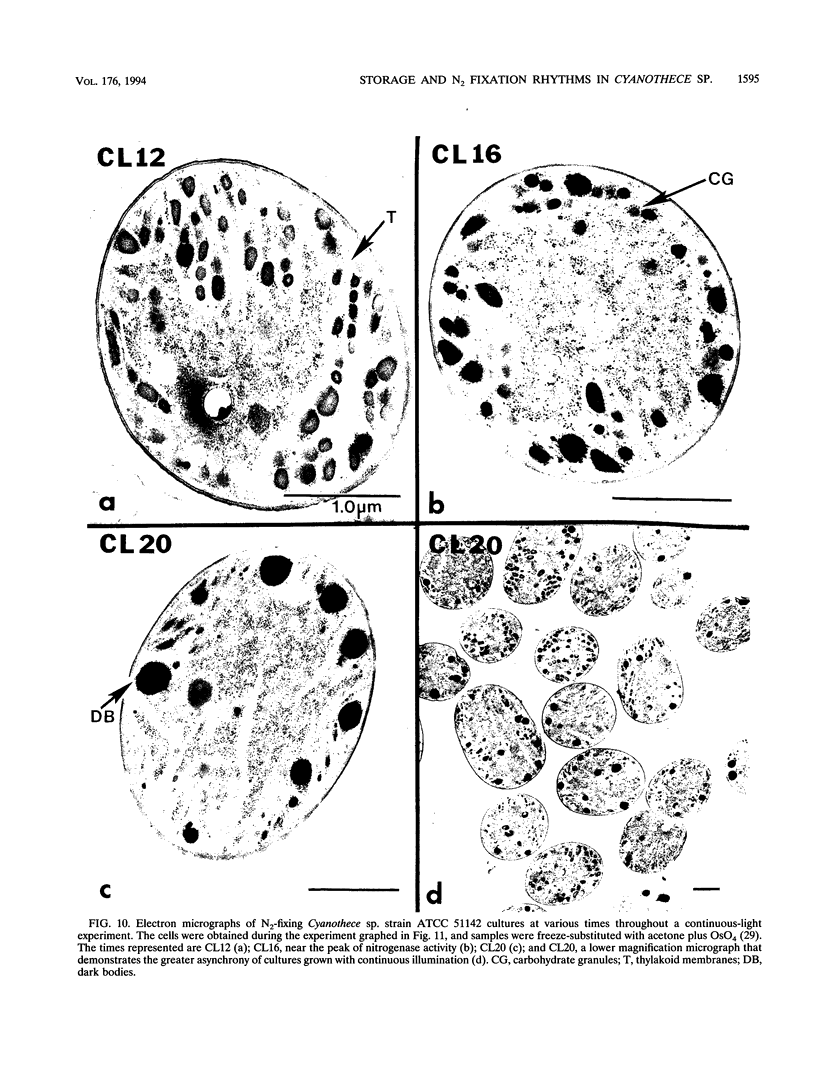
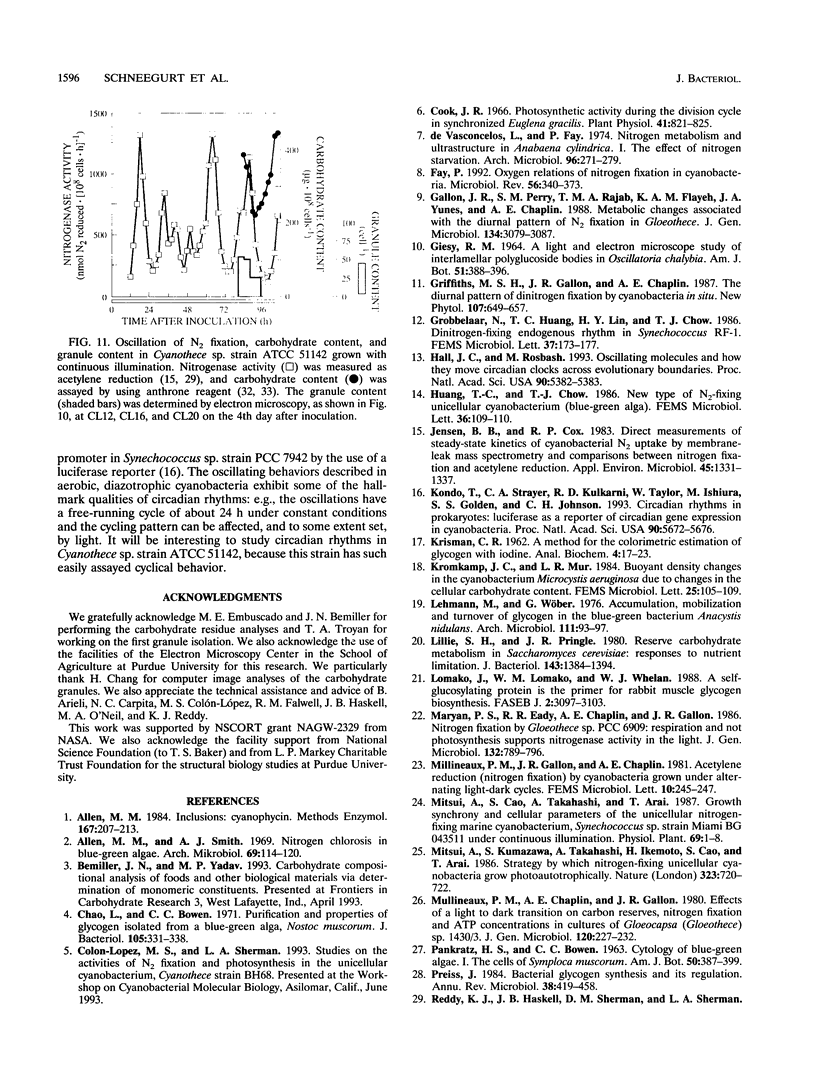
It has been shown that some aerobic, unicellular, diazotrophic cyanobacteria temporally separate photosynthetic O2 evolution and oxygen-sensitive N2 fixation. Cyanothece sp. ATCC strain 51142 is an aerobic, unicellular, diazotrophic cyanobacterium that fixes N2 during discrete periods of its cell cycle. When the bacteria are maintained under diurnal light-dark cycles, N2 fixation occurs in the dark. Similar cycling is observed in continuous light, implicating a circadian rhythm. Under N2-fixing conditions, large inclusion granules form between the thylakoid membranes. Maximum granulation, as observed by electron microscopy, occurs before the onset of N2 fixation, and the granules decrease in number during the period of N2 fixation. The granules can be purified from cell homogenates by differential centrifugation. Biochemical analyses of the granules indicate that these structures are primarily carbohydrate, with some protein. Further analyses of the carbohydrate have shown that it is a glucose polymer with some characteristics of glycogen. It is proposed that N2 fixation is driven by energy and reducing power stored in these inclusion granules. Cyanothece sp. strain ATCC 51142 represents an excellent experimental organism for the study of the protective mechanisms of nitrogenase, metabolic events in cyanobacteria under normal and stress conditions, the partitioning of resources between growth and storage, and biological rhythms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Chao L., Bowen C. C. Purification and properties of glycogen isolated from a blue-green alga, Nostoc muscorum. J Bacteriol. 1971 Jan;105(1):331–338. doi: 10.1128/jb.105.1.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. R. Photosynthetic activity during the division cycle in synchronized Euglena gracilis. Plant Physiol. 1966 May;41(5):821–825. doi: 10.1104/pp.41.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Oscillating molecules and how they move circadian clocks across evolutionary boundaries. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5382–5383. doi: 10.1073/pnas.90.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B. B., Cox R. P. Direct measurements of steady-state kinetics of cyanobacterial n(2) uptake by membrane-leak mass spectrometry and comparisons between nitrogen fixation and acetylene reduction. Appl Environ Microbiol. 1983 Apr;45(4):1331–1337. doi: 10.1128/aem.45.4.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISMAN C. R. A method for the colorimetric estimation of glycogen with iodine. Anal Biochem. 1962 Jul;4:17–23. doi: 10.1016/0003-2697(62)90014-3. [DOI] [PubMed] [Google Scholar]
- Kondo T., Strayer C. A., Kulkarni R. D., Taylor W., Ishiura M., Golden S. S., Johnson C. H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomako J., Lomako W. M., Whelan W. J. A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis. FASEB J. 1988 Dec;2(15):3097–3103. doi: 10.1096/fasebj.2.15.2973423. [DOI] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P. Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen beta-particles isolated by the precipitation-centrifugation method. J Cell Biol. 1968 Jul;38(1):130–150. doi: 10.1083/jcb.38.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]
- van Eykelenburg C. Ecophysiological studies on Spirulina platensis. Effect of temperature, light intensity and nitrate concentration on growth and ultrastructure. Antonie Van Leeuwenhoek. 1980;46(2):113–127. doi: 10.1007/BF00444067. [DOI] [PubMed] [Google Scholar]