Abstract
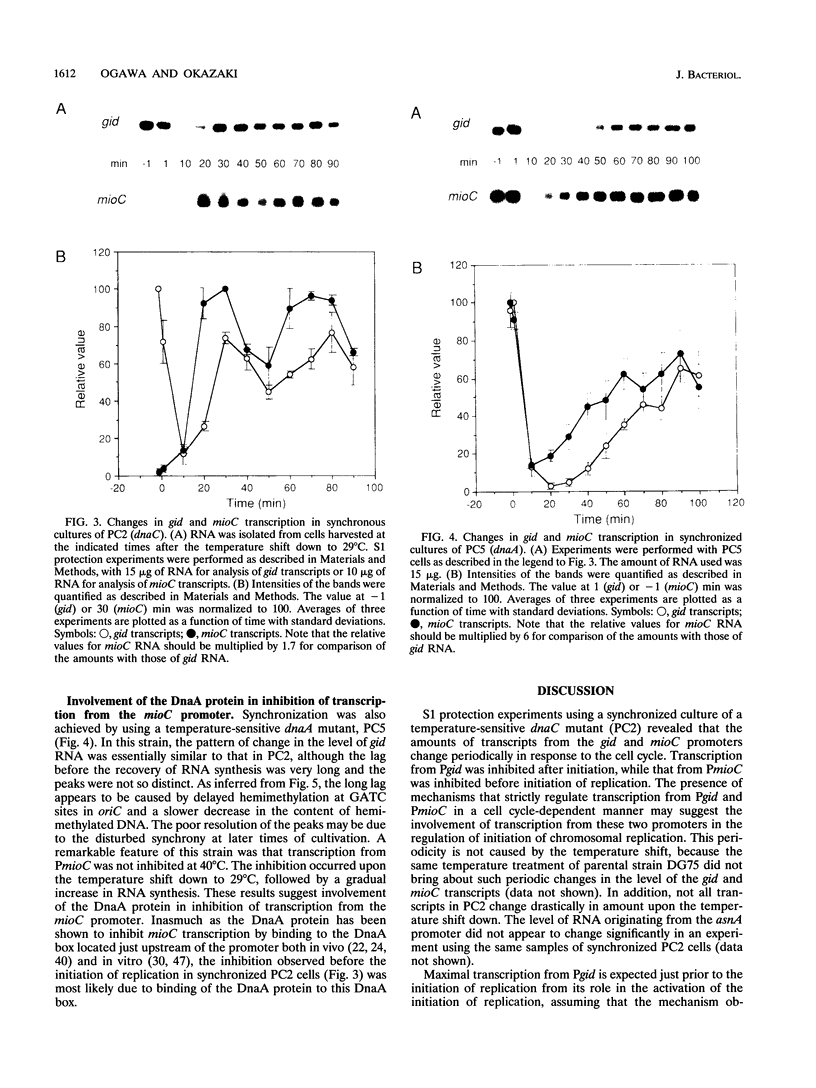
Transcription from the gid and mioC promoters, which neighbor the origin of replication of the Escherichia coli chromosome (oriC), has been implicated in the control of initiation of replication of minichromosomes. The amounts of transcripts from these two promoters on the chromosome were quantified at various times in a synchronized culture of a temperature-sensitive dnaC mutant strain. Transcription from the gid promoter was most active before the initiation of replication and was inhibited after initiation, during the time corresponding to the period of sequestration of the oriC region from the dam methyltransferase. On the other hand, transcription from the mioC promoter was inhibited before initiation and the inhibition was relieved after initiation prior to the recovery of gid transcription. The strict regulation of transcription from the gid and mioC promoters may be involved in positive and negative control of chromosomal replication, respectively, as has been suggested for minichromosome replication. The DnaA protein was involved in repression of mioC transcription, indicating that the activity of the DnaA protein changes during the cell cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Asai T., Chen C. P., Nagata T., Takanami M., Imai M. Transcription in vivo within the replication origin of the Escherichia coli chromosome: a mechanism for activating initiation of replication. Mol Gen Genet. 1992 Jan;231(2):169–178. doi: 10.1007/BF00279788. [DOI] [PubMed] [Google Scholar]
- Asai T., Takanami M., Imai M. The AT richness and gid transcription determine the left border of the replication origin of the E. coli chromosome. EMBO J. 1990 Dec;9(12):4065–4072. doi: 10.1002/j.1460-2075.1990.tb07628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Kornberg A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: an RNA-DNA hybrid near oriC. Cell. 1988 Oct 7;55(1):113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Chiaramello A. E., Zyskind J. W. Expression of Escherichia coli dnaA and mioC genes as a function of growth rate. J Bacteriol. 1989 Aug;171(8):4272–4280. doi: 10.1128/jb.171.8.4272-4280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Ross W., Wild J., Gourse R. L. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol. 1992 Jan;174(2):398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Egan J. B., Roth A., Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991 Aug 11;19(15):4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker D. E., Jr, Rokeach L. A., Ganea D., Chiaramello A., Zyskind J. W. Transcription termination within the Escherichia coli origin of DNA replication, oriC. Mol Gen Genet. 1986 Apr;203(1):101–109. doi: 10.1007/BF00330390. [DOI] [PubMed] [Google Scholar]
- Kano Y., Ogawa T., Ogura T., Hiraga S., Okazaki T., Imamoto F. Participation of the histone-like protein HU and of IHF in minichromosomal maintenance in Escherichia coli. Gene. 1991 Jul 15;103(1):25–30. doi: 10.1016/0378-1119(91)90386-p. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Leonard A. C., Whitford W. G., Helmstetter C. E. Involvement of DNA superhelicity in minichromosome maintenance in Escherichia coli. J Bacteriol. 1985 Feb;161(2):687–695. doi: 10.1128/jb.161.2.687-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A., Vannier F., Dehbi M., Henckes G., Séror S. J. The stringent response blocks DNA replication outside the ori region in Bacillus subtilis and at the origin in Escherichia coli. J Mol Biol. 1991 Jun 20;219(4):605–613. doi: 10.1016/0022-2836(91)90657-r. [DOI] [PubMed] [Google Scholar]
- Lother H., Kölling R., Kücherer C., Schauzu M. dnaA protein-regulated transcription: effects on the in vitro replication of Escherichia coli minichromosomes. EMBO J. 1985 Feb;4(2):555–560. doi: 10.1002/j.1460-2075.1985.tb03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Messer W. Promoters in the E. coli replication origin. Nature. 1981 Nov 26;294(5839):376–378. doi: 10.1038/294376a0. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Atlung T., Rasmussen K. V. Stability and replication control of Escherichia coli minichromosomes. J Bacteriol. 1987 Jun;169(6):2835–2842. doi: 10.1128/jb.169.6.2835-2842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A., Boye E. Different effects of mioC transcription on initiation of chromosomal and minichromosomal replication in Escherichia coli. Nucleic Acids Res. 1992 Jun 25;20(12):3029–3036. doi: 10.1093/nar/20.12.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki N., Okazaki T., Ogawa T. In vitro transcription of the origin region of replication of the Escherichia coli chromosome. J Biol Chem. 1988 Oct 5;263(28):14176–14183. [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. Concurrent transcription from the gid and mioC promoters activates replication of an Escherichia coli minichromosome. Mol Gen Genet. 1991 Nov;230(1-2):193–200. doi: 10.1007/BF00290668. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Wada M., Kano Y., Imamoto F., Okazaki T. DNA replication in Escherichia coli mutants that lack protein HU. J Bacteriol. 1989 Oct;171(10):5672–5679. doi: 10.1128/jb.171.10.5672-5679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Kassavetis G. A., Zyskind J. W. RNA polymerase pauses in vitro within the Escherichia coli origin of replication at the same sites where termination occurs in vivo. J Biol Chem. 1987 May 25;262(15):7264–7272. [PubMed] [Google Scholar]
- Sakakibara Y., Yuasa S. Continuous synthesis of the dnaA gene product of Escherichia coli in the cell cycle. Mol Gen Genet. 1982;186(1):87–94. doi: 10.1007/BF00422917. [DOI] [PubMed] [Google Scholar]
- Schauzu M. A., Kücherer C., Kölling R., Messer W., Lother H. Transcripts within the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1987 Mar 25;15(6):2479–2497. doi: 10.1093/nar/15.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987 Jul 17;50(2):259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- Stuitje A. R., Meijer M. Maintenance and incompatibility of plasmids carrying the replication origin of the Escherichia coli chromosome: evidence for a control region of replication between oriC and asnA. Nucleic Acids Res. 1983 Aug 25;11(16):5775–5791. doi: 10.1093/nar/11.16.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., de Wind N., van der Spek J. C., Pors T. H., Meijer M. Dissection of promoter sequences involved in transcriptional activation of the Escherichia coli replication origin. Nucleic Acids Res. 1986 Mar 11;14(5):2333–2344. doi: 10.1093/nar/14.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Hiraga S. Negative control of oriC plasmid replication by transcription of the oriC region. Mol Gen Genet. 1985;200(1):21–26. doi: 10.1007/BF00383307. [DOI] [PubMed] [Google Scholar]
- Theisen P. W., Grimwade J. E., Leonard A. C., Bogan J. A., Helmstetter C. E. Correlation of gene transcription with the time of initiation of chromosome replication in Escherichia coli. Mol Microbiol. 1993 Nov;10(3):575–584. doi: 10.1111/j.1365-2958.1993.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Wang Q. P., Kaguni J. M. Transcriptional repression of the dnaA gene of Escherichia coli by dnaA protein. Mol Gen Genet. 1987 Oct;209(3):518–525. doi: 10.1007/BF00331158. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Weinberger M., Helmstetter C. E. Inhibition of protein synthesis transiently stimulates initiation of minichromosome replication in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3591–3596. doi: 10.1128/jb.171.7.3591-3596.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. DNA replication, the bacterial cell cycle, and cell growth. Cell. 1992 Apr 3;69(1):5–8. doi: 10.1016/0092-8674(92)90112-p. [DOI] [PubMed] [Google Scholar]
- de Wind N., Parren P., Stuitje A. R., Meijer M. Evidence for the involvement of the 16kD gene promoter in initiation of chromosomal replication of Escherichia coli strains carrying a B/r-derived replication origin. Nucleic Acids Res. 1987 Jun 25;15(12):4901–4914. doi: 10.1093/nar/15.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]