Abstract
The perfringolysin O (theta-toxin) gene (pfoA) of Clostridium perfringens was cloned into an Escherichia coli-C. perfringens shuttle vector, and the pfoA gene was expressed in mutants of C. perfringens 13 which lacked the production of perfringolysin O. One group (SI117) could express the pfoA gene, and the other (SI112) could not. A mutation in the regulatory system for pfoA gene expression was suspected in SI112. A chromosomal DNA library constructed from strain 13 was transformed into strain SI112 to identify the regulatory gene(s) for the pfoA gene. Five strains of 10,000 transformants restored perfringolysin O production. All contained a 2.5-kb DNA fragment. This fragment activated the transcription of the pfoA gene and also restored the production of collagenase (kappa-toxin) and hemagglutinin in strain SI112. Deletion analysis showed that a 1.25-kb region was sufficient for the trans activity, and sequence analysis disclosed that open reading frame 2 (ORF2) was located in this region. A homology search for the deduced amino acid sequence revealed that ORF2 was homologous to a response regulator in a two-component signal transduction system. ORF2 was designated virR, and it is suggested that the virR gene plays an important role in the pathogenicity of C. perfringens.
Full text
PDF



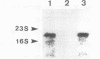
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. P., Blaschek H. P. Electroporation-induced transformation of intact cells of Clostridium perfringens. Appl Environ Microbiol. 1988 Sep;54(9):2322–2324. doi: 10.1128/aem.54.9.2322-2324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. P., Blaschek H. P. Factors involved in the electroporation-induced transformation of Clostridium perfringens. FEMS Microbiol Lett. 1990 Jul;58(2):217–220. doi: 10.1111/j.1574-6968.1990.tb13981.x. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Deretic V., Dikshit R., Konyecsni W. M., Chakrabarty A. M., Misra T. K. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol. 1989 Mar;171(3):1278–1283. doi: 10.1128/jb.171.3.1278-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garnier T., Cole S. T. Complete nucleotide sequence and genetic organization of the bacteriocinogenic plasmid, pIP404, from Clostridium perfringens. Plasmid. 1988 Mar;19(2):134–150. doi: 10.1016/0147-619x(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Hatheway C. L. Toxigenic clostridia. Clin Microbiol Rev. 1990 Jan;3(1):66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Higashi Y. An activity which restores theta toxin activity in some theta toxin-deficient mutants of Clostridium perfringens. Microbiol Immunol. 1992;36(5):523–527. doi: 10.1111/j.1348-0421.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Tatsuki T., Higashi Y., Amano T. Complementation characteristics of newly isolated mutants from two groups of strains of Clostridium perfringens. Biken J. 1981 Jun;24(1-2):13–21. [PubMed] [Google Scholar]
- Katayama S., Matsushita O., Minami J., Mizobuchi S., Okabe A. Comparison of the alpha-toxin genes of Clostridium perfringens type A and C strains: evidence for extragenic regulation of transcription. Infect Immun. 1993 Feb;61(2):457–463. doi: 10.1128/iai.61.2.457-463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony D. E., Moore T. I. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can J Microbiol. 1976 Jul;22(7):953–959. doi: 10.1139/m76-138. [DOI] [PubMed] [Google Scholar]
- McDonel J. L. Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol Ther. 1980;10(3):617–655. doi: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Okabe A., Shimizu T., Hayashi H. Cloning and sequencing of a phospholipase C gene of Clostridium perfringens. Biochem Biophys Res Commun. 1989 Apr 14;160(1):33–39. doi: 10.1016/0006-291x(89)91616-1. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Holmes W. M., Hylemon P. B. Modified plasmid isolation method for Clostridium perfringens and Clostridium absonum. Appl Environ Microbiol. 1986 Jul;52(1):197–199. doi: 10.1128/aem.52.1.197-199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Cole S. T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991 Dec;55(4):621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Okabe A., Minami J., Hayashi H. An upstream regulatory sequence stimulates expression of the perfringolysin O gene of Clostridium perfringens. Infect Immun. 1991 Jan;59(1):137–142. doi: 10.1128/iai.59.1.137-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan J., Warner T. A., Scott P. T., Bannam T. L., Berryman D. I., Rood J. I. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid. 1992 May;27(3):207–219. doi: 10.1016/0147-619x(92)90023-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga T., Matsushita O., Katayama S., Minami J., Okabe A. Role of the upstream region containing an intrinsic DNA curvature in the negative regulation of the phospholipase C gene of Clostridium perfringens. Microbiol Immunol. 1992;36(6):603–613. doi: 10.1111/j.1348-0421.1992.tb02060.x. [DOI] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y., Ito A., Sato H. Theta-toxin of Clostridium perfringens. I. Purification and some properties. Biochim Biophys Acta. 1977 Oct 26;494(2):301–313. doi: 10.1016/0005-2795(77)90159-3. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]