Abstract
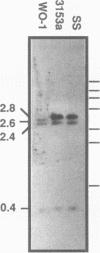
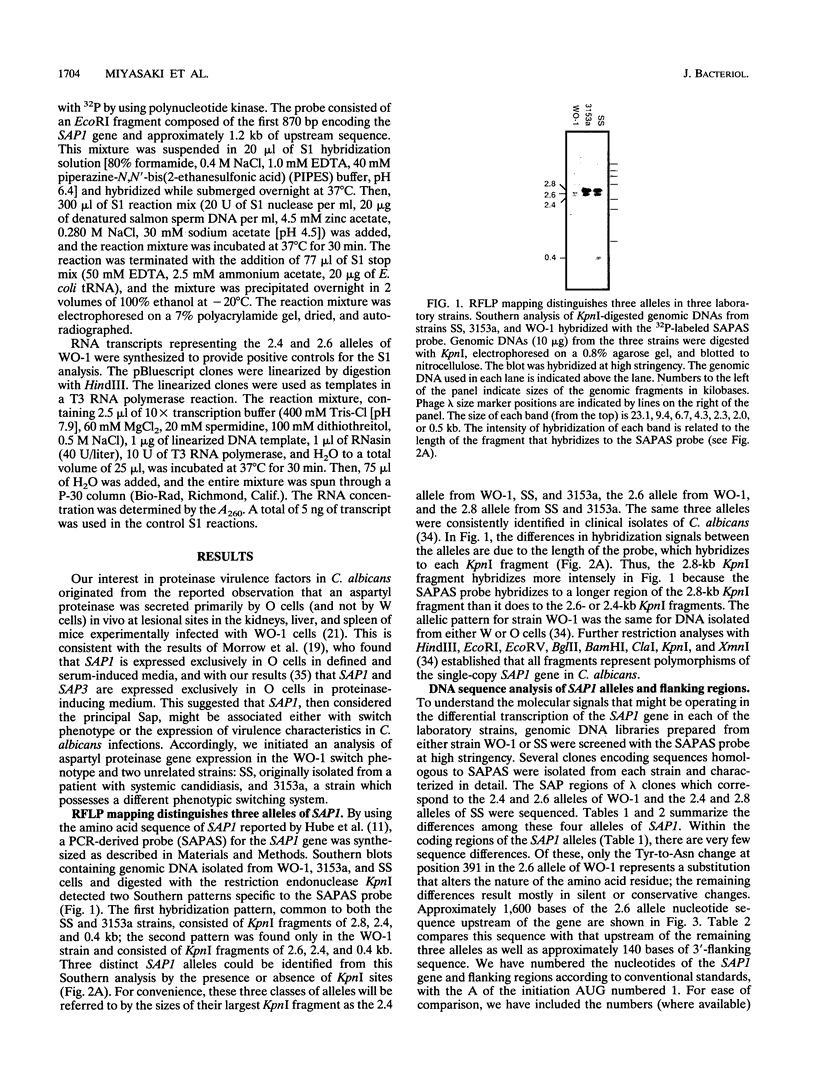
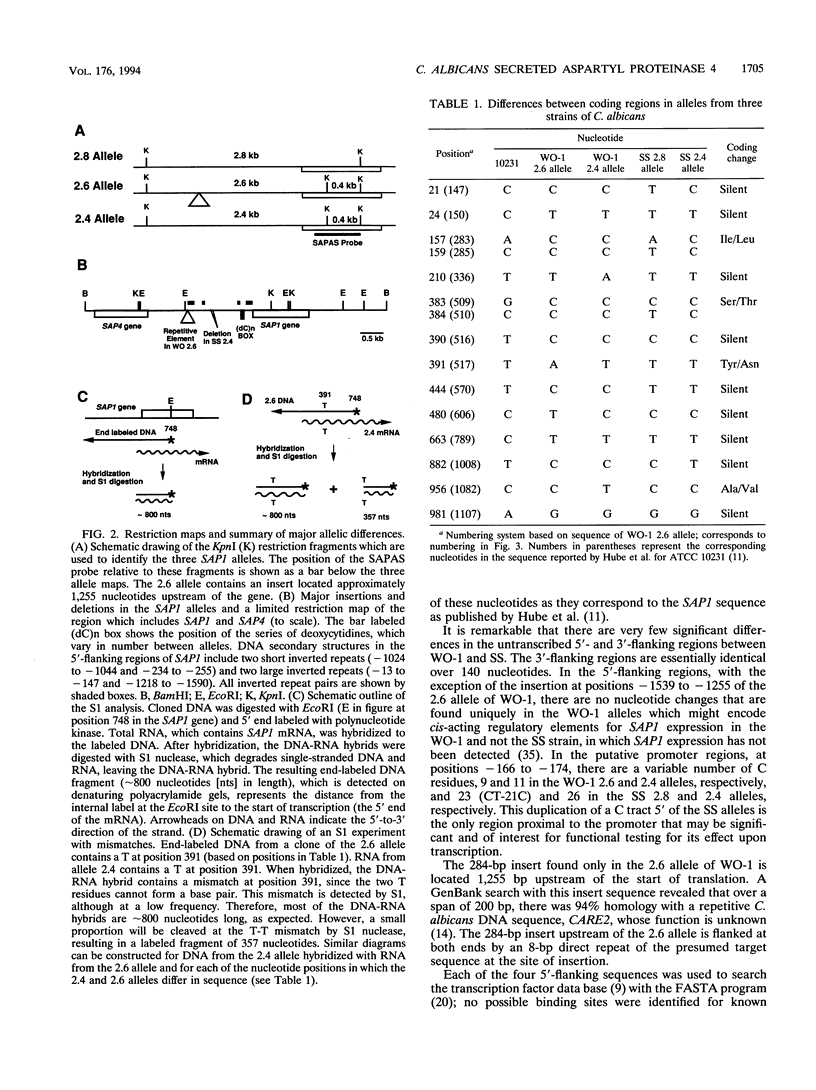
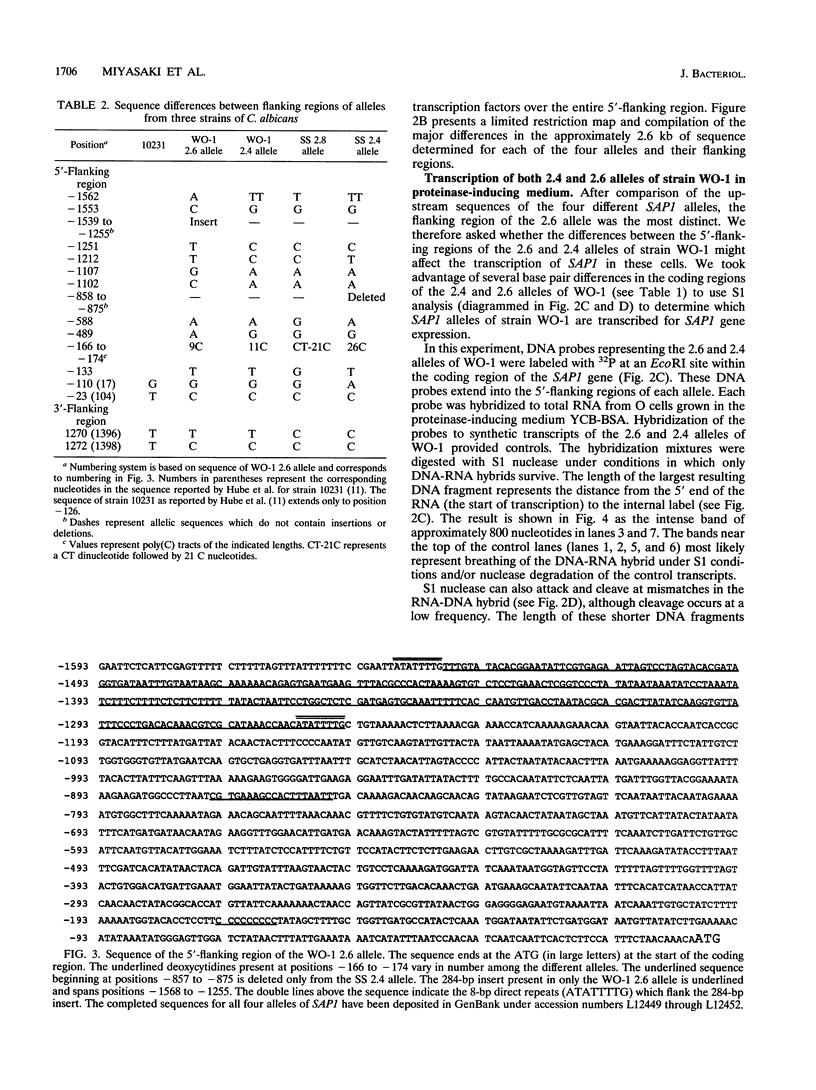
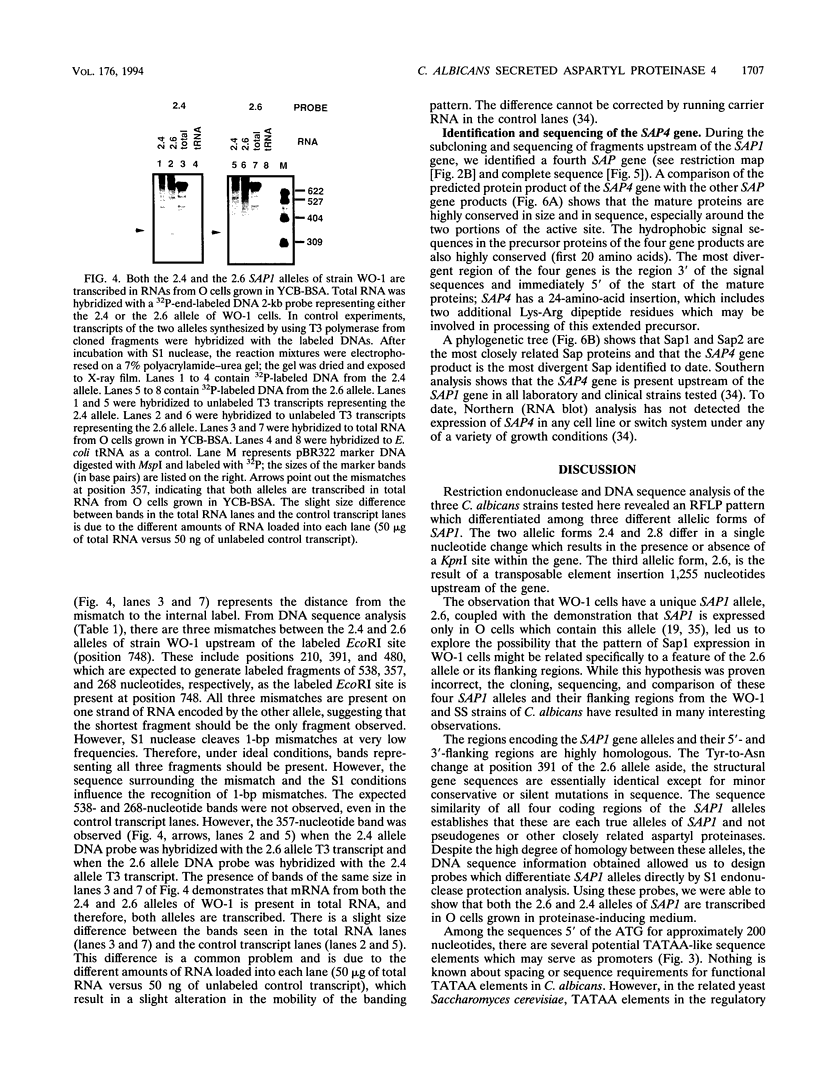
Candida albicans secreted aspartyl proteinases (Sap), products of the SAP genes, which are presumed to act as virulence factors. In the C. albicans strain WO-1, the ability to secrete Sap1 is regulated with switch phenotype, another putative virulence factor. KpnI restriction fragment length polymorphisms differentiate between several distinct SAP1 alleles in laboratory and clinical strains. Both SAP1 alleles from strain WO-1 along with their 5'- and 3'-flanking regions were cloned and sequenced, as were both alleles from another strain, SS. The 5'-flanking regions were remarkably similar in all four of the sequenced alleles over approximately 1,500 nucleotides. S1 analysis revealed that both alleles of WO-1 are transcribed. Characterization of the one allele from strain WO-1 identified a 284-nucleotide insertion flanked by 8-bp direct repeats that shows homology to the CARE2 repetitive element and that is not present in the other alleles. Characterization of the SAP1 alleles also identified a fourth SAP gene (SAP4) that includes an extended leader sequence. SAP4 is positioned upstream, in tandem to SAP1, in all strains tested and may encode another closely related secreted aspartyl proteinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bernards A., Van der Ploeg L. H., Frasch A. C., Borst P., Boothroyd J. C., Coleman S., Cross G. A. Activation of trypanosome surface glycoprotein genes involves a duplication-transposition leading to an altered 3' end. Cell. 1981 Dec;27(3 Pt 2):497–505. doi: 10.1016/0092-8674(81)90391-3. [DOI] [PubMed] [Google Scholar]
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Pabich E. K., Donahue T. F. Mutational analysis of the HIS4 translational initiator region in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2964–2975. doi: 10.1128/mcb.8.7.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Ghannoum M., Abu Elteen K. Correlative relationship between proteinase production, adherence and pathogenicity of various strains of Candida albicans. J Med Vet Mycol. 1986 Oct;24(5):407–413. doi: 10.1080/02681218680000621. [DOI] [PubMed] [Google Scholar]
- Ghosh D. TFD: the transcription factors database. Nucleic Acids Res. 1992 May 11;20 (Suppl):2091–2093. doi: 10.1093/nar/20.suppl.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Yoshiura K., Negi M., Ogawa H. Keratinolytic proteinase produced by Candida albicans. Sabouraudia. 1984;22(3):175–183. [PubMed] [Google Scholar]
- Hube B., Turver C. J., Odds F. C., Eiffert H., Boulnois G. J., Köchel H., Rüchel R. Sequence of the Candida albicans gene encoding the secretory aspartate proteinase. J Med Vet Mycol. 1991;29(2):129–132. [PubMed] [Google Scholar]
- Kaminishi H., Hagihara Y., Hayashi S., Cho T. Isolation and characteristics of collagenolytic enzyme produced by Candida albicans. Infect Immun. 1986 Aug;53(2):312–316. doi: 10.1128/iai.53.2.312-316.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker B. A., Page L. S., Lott T. J., Kobayashi G. S. Isolation, characterization, and sequencing of Candida albicans repetitive element 2. Gene. 1992 Jul 1;116(1):51–57. doi: 10.1016/0378-1119(92)90628-3. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Magee B. B., Hube B., Wright R. J., Sullivan P. J., Magee P. T. The genes encoding the secreted aspartyl proteinases of Candida albicans constitute a family with at least three members. Infect Immun. 1993 Aug;61(8):3240–3243. doi: 10.1128/iai.61.8.3240-3243.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. J., Hurst S. F., Bragg S. L., Kuykendall R. J., Diaz H., Pohl J., Reiss E. Heterogeneity of the purified extracellular aspartyl proteinase from Candida albicans: characterization with monoclonal antibodies and N-terminal amino acid sequence analysis. Infect Immun. 1993 May;61(5):2030–2036. doi: 10.1128/iai.61.5.2030-2036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B., Srikantha T., Anderson J., Soll D. R. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect Immun. 1993 May;61(5):1823–1828. doi: 10.1128/iai.61.5.1823-1828.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B., Srikantha T., Soll D. R. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992 Jul;12(7):2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D., Morrow B. J. Candida albicans acid proteinase: characterization and role in candidiasis. Adv Exp Med Biol. 1991;306:173–183. doi: 10.1007/978-1-4684-6012-4_22. [DOI] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Tegeler R., Trost M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia. 1982 Sep;20(3):233–244. doi: 10.1080/00362178285380341. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Uhlemann K., Böning B. Secretion of acid proteinases by different species of the genus Candida. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):537–548. [PubMed] [Google Scholar]
- Rüchel R., de Bernardis F., Ray T. L., Sullivan P. A., Cole G. T. Candida acid proteinases. J Med Vet Mycol. 1992;30 (Suppl 1):123–132. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992 Apr;5(2):183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Morrow B., Srikantha T. High-frequency phenotypic switching in Candida albicans. Trends Genet. 1993 Feb;9(2):61–65. doi: 10.1016/0168-9525(93)90189-O. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia. 1965 Oct;4(3):187–193. doi: 10.1080/00362176685190421. [DOI] [PubMed] [Google Scholar]
- White T. C., Miyasaki S. H., Agabian N. Three distinct secreted aspartyl proteinases in Candida albicans. J Bacteriol. 1993 Oct;175(19):6126–6133. doi: 10.1128/jb.175.19.6126-6133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. J., Carne A., Hieber A. D., Lamont I. L., Emerson G. W., Sullivan P. A. A second gene for a secreted aspartate proteinase in Candida albicans. J Bacteriol. 1992 Dec;174(23):7848–7853. doi: 10.1128/jb.174.23.7848-7853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]