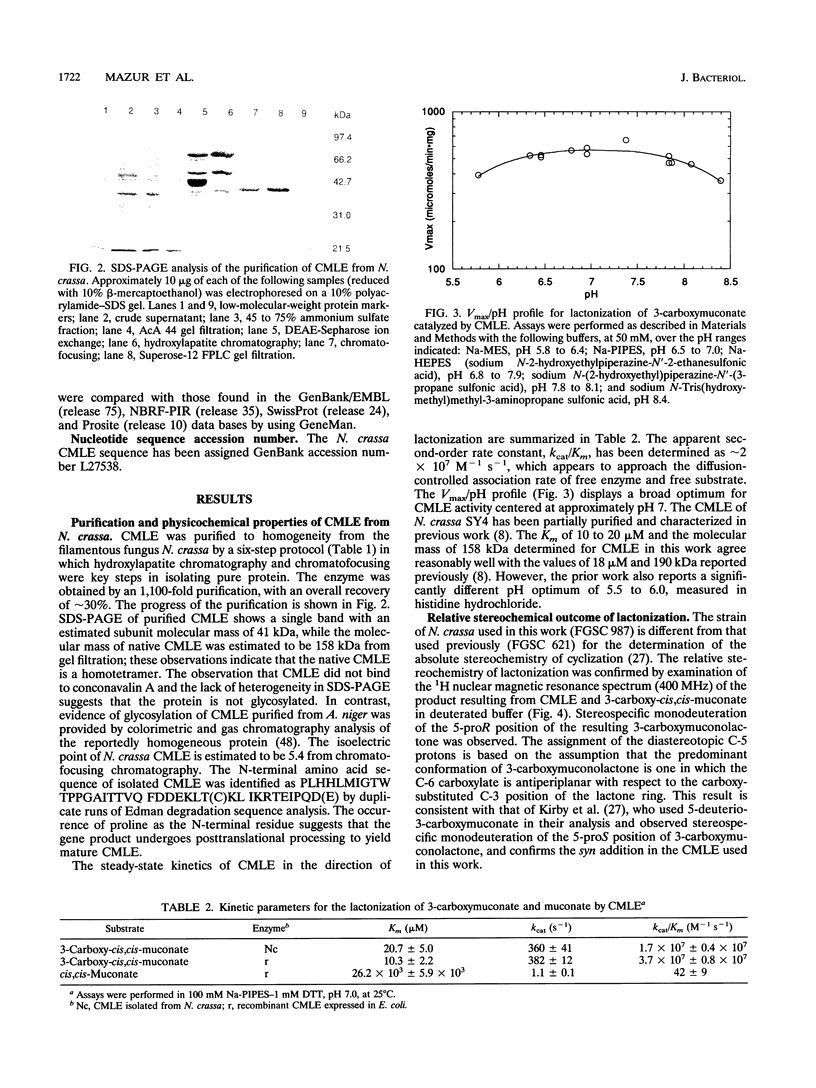
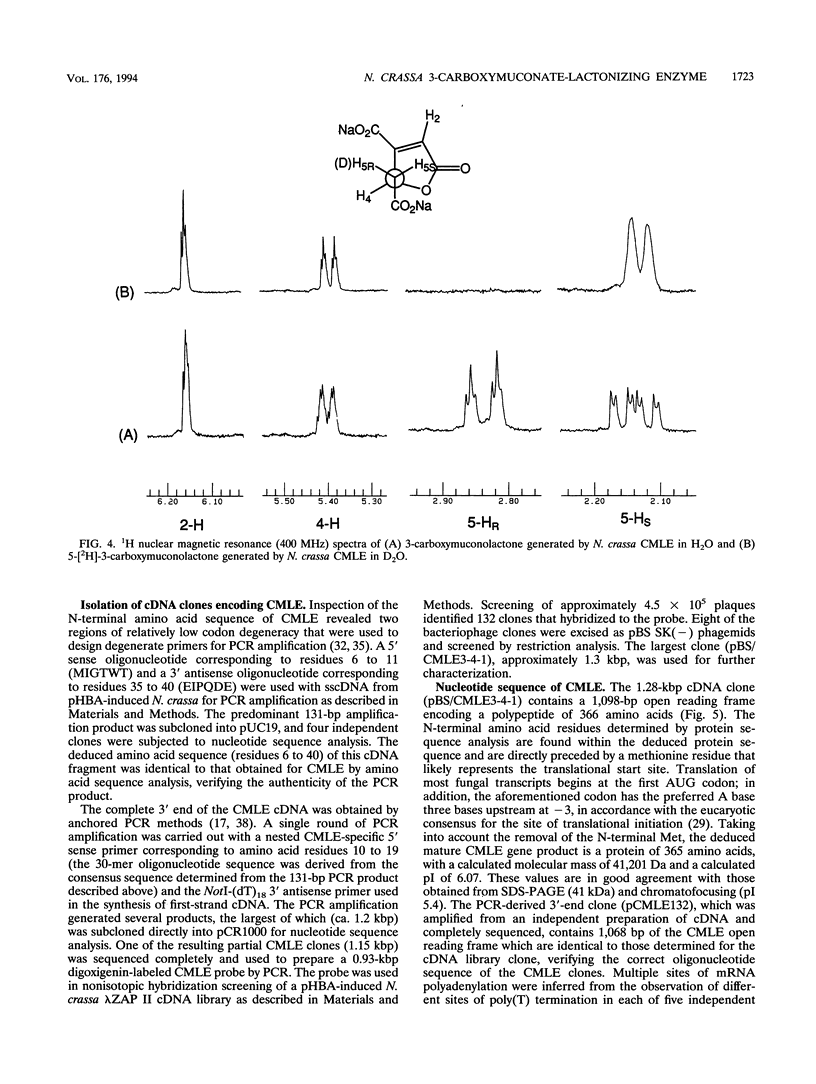
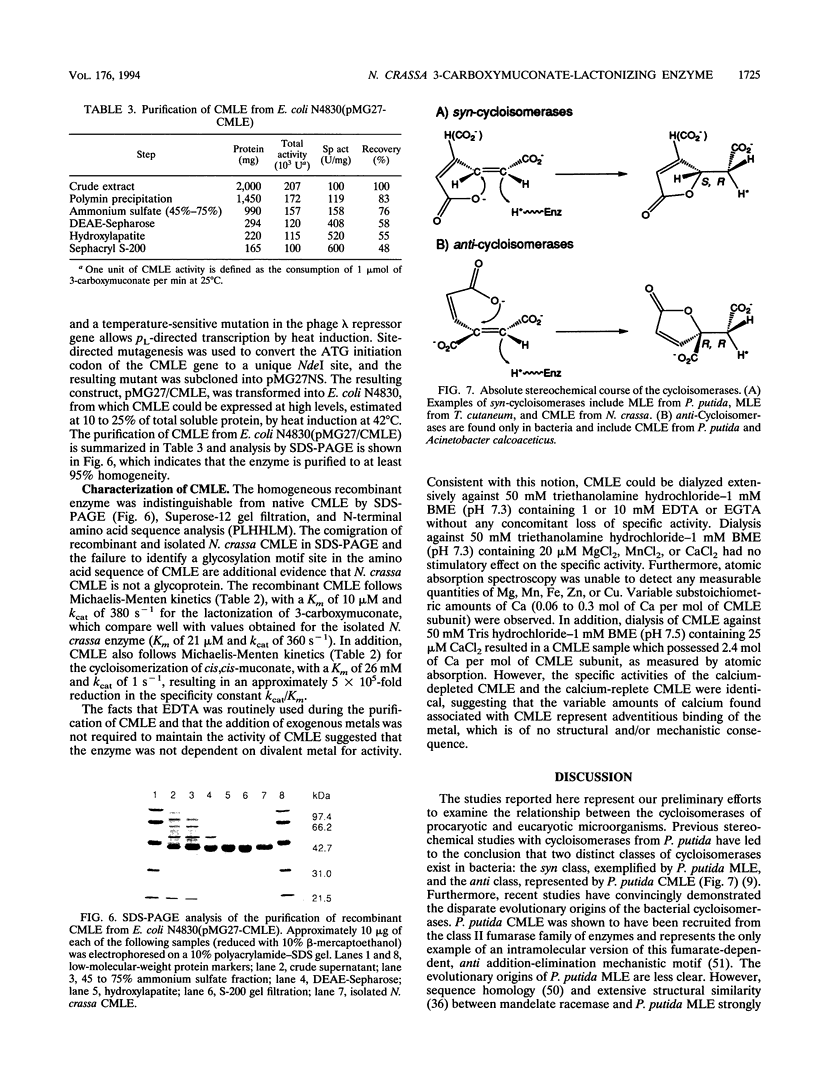
Abstract
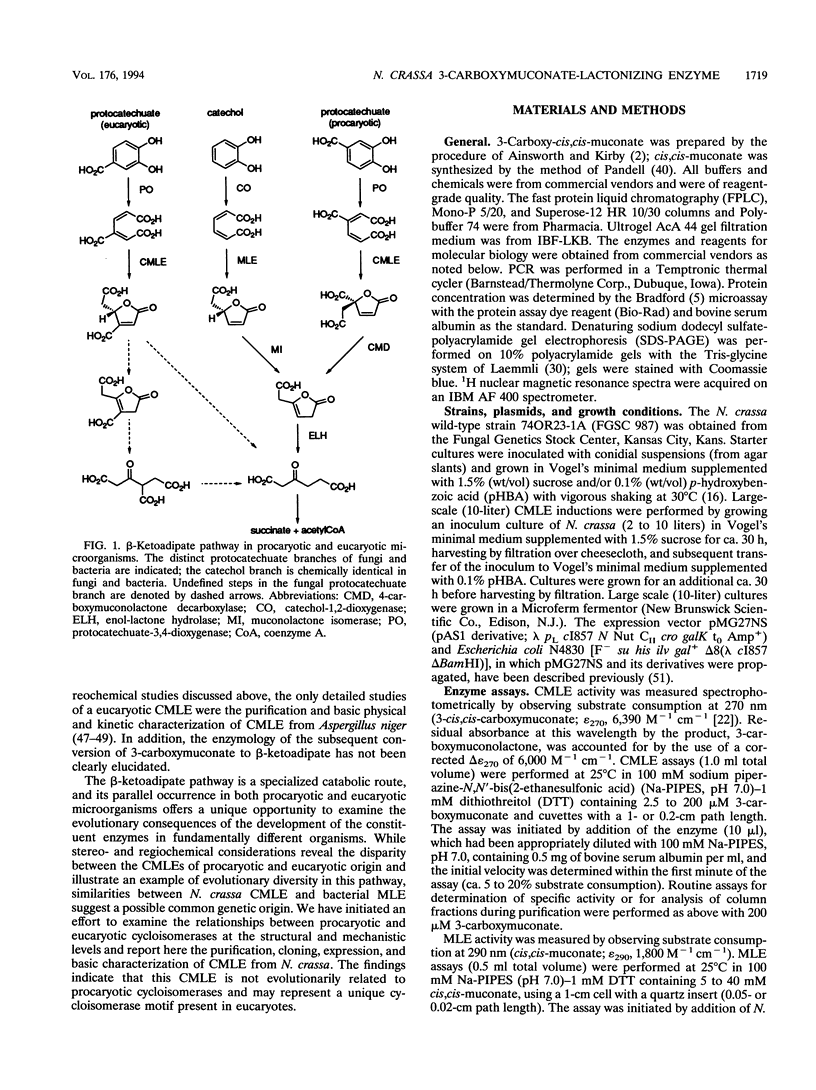
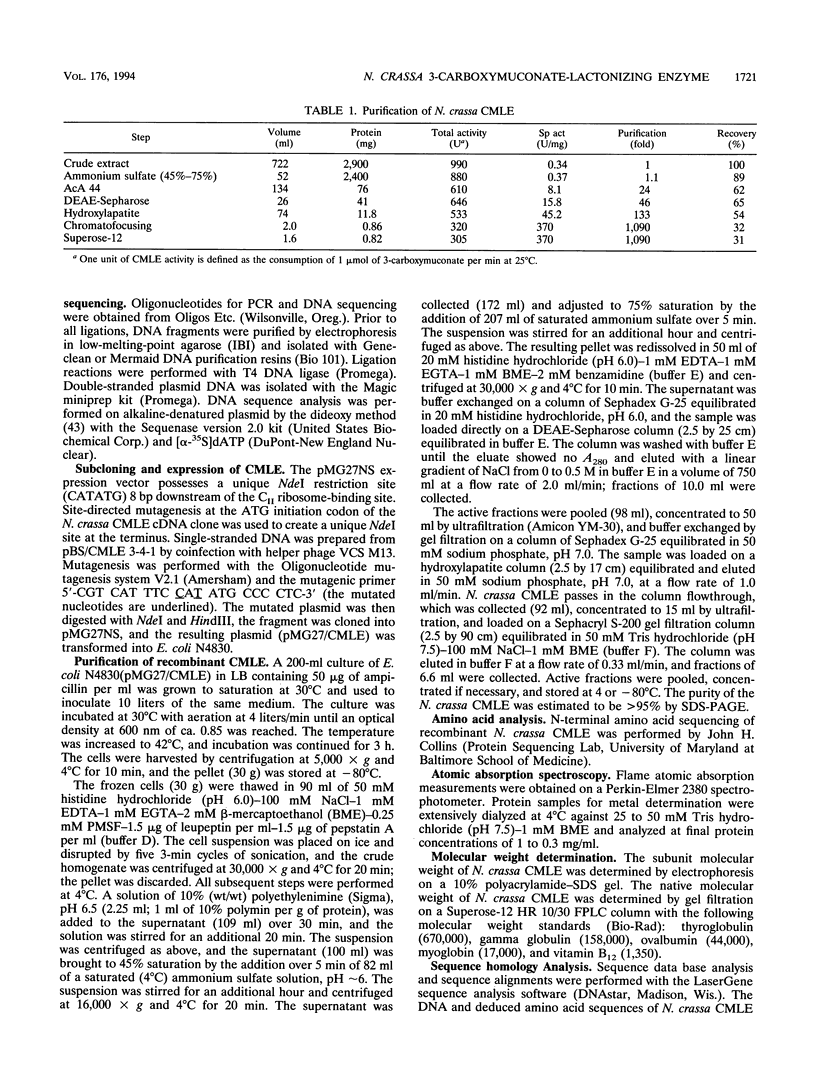
3-Carboxy-cis,cis-muconate lactonizing enzyme (CMLE; EC 5.5.1.5) from Neurospora crassa catalyzes the reversible gamma-lactonization of 3-carboxy-cis,cis-muconate by a syn-1,2 addition-elimination reaction. The stereochemical and regiochemical course of the reaction is (i) opposite that of CMLE from Pseudomonas putida (EC 5.5.1.2) and (ii) identical to that of cis,cis-muconate lactonizing enzyme (MLE; EC 5.5.1.1) from P. putida. In order to determine the mechanistic and evolutionary relationships between N. crassa CMLE and the procaryotic cycloisomerases, we have purified CMLE from N. crassa to homogeneity and determined its nucleotide sequence from a cDNA clone isolated from a p-hydroxybenzoate-induced N. crassa cDNA library. The deduced amino acid sequence predicts a protein of 41.2 kDa (365 residues) which does not exhibit sequence similarity with any of the bacterial cycloisomerases. The cDNA encoding N. crassa CMLE was expressed in Escherichia coli, and the purified recombinant protein exhibits physical and kinetic properties equivalent to those found for the isolated N. crassa enzyme. We also report that N. crassa CMLE possesses substantially reduced yet significant levels of MLE activity with cis,cis-muconate and, furthermore, does not appear to be dependent on divalent metals for activity. These data suggest that the N. crassa CMLE may represent a novel eucaryotic motif in the cycloisomerase enzyme family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimi J., Badylak J., Williams J., Chen Z. D., Zalkin H., Dixon J. E. Cloning of a cDNA encoding adenylosuccinate lyase by functional complementation in Escherichia coli. J Biol Chem. 1990 Jun 5;265(16):9011–9014. [PubMed] [Google Scholar]
- Aldrich T. L., Frantz B., Gill J. F., Kilbane J. J., Chakrabarty A. M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52(2-3):185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cronin C. N., Kirsch J. F. Role of arginine-292 in the substrate specificity of aspartate aminotransferase as examined by site-directed mutagenesis. Biochemistry. 1988 Jun 14;27(12):4572–4579. doi: 10.1021/bi00412a052. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., GAFFORD R. D., TATUM E. L. The metabolism of protocatechuic acid by Neurospora. J Biol Chem. 1956 Apr;219(2):781–796. [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. cis,cis-Muconate cyclase from Trichosporon cutaneum. Biochem J. 1980 Oct 1;191(1):37–43. doi: 10.1042/bj1910037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A., Ollis D. L., Steitz T. A. Crystal structure of muconate lactonizing enzyme at 3 A resolution. J Mol Biol. 1987 Mar 5;194(1):143–153. doi: 10.1016/0022-2836(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Kuramitsu S., Inoue Y., Morino Y., Kagamiyama H. [Arg292----Val] or [Arg292----Leu] mutation enhances the reactivity of Escherichia coli aspartate aminotransferase with aromatic amino acids. Biochem Biophys Res Commun. 1989 Feb 28;159(1):337–342. doi: 10.1016/0006-291x(89)92443-1. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M. Preparation of extracts from yeast. Methods Enzymol. 1990;182:154–174. doi: 10.1016/0076-6879(90)82015-t. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzillo J. J. Chemiluminescent nucleic acid detection with digoxigenin-labeled probes: a model system with probes for angiotensin converting enzyme which detect less than one attomole of target DNA. Anal Biochem. 1991 Apr;194(1):45–53. doi: 10.1016/0003-2697(91)90149-n. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Mazur P., Pieken W. A., Budihas S. R., Williams S. E., Wong S., Kozarich J. W. Cis,cis-muconate lactonizing enzyme from Trichosporon cutaneum: evidence for a novel class of cycloisomerases in eucaryotes. Biochemistry. 1994 Feb 22;33(7):1961–1970. doi: 10.1021/bi00173a045. [DOI] [PubMed] [Google Scholar]
- Moremen K. W. Isolation of a rat liver Golgi mannosidase II clone by mixed oligonucleotide-primed amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5276–5280. doi: 10.1073/pnas.86.14.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhart D. J., Kenyon G. L., Gerlt J. A., Petsko G. A. Mandelate racemase and muconate lactonizing enzyme are mechanistically distinct and structurally homologous. Nature. 1990 Oct 18;347(6294):692–694. doi: 10.1038/347692a0. [DOI] [PubMed] [Google Scholar]
- Ngai K. L., Ornston L. N., Kallen R. G. Enzymes of the beta-ketoadipate pathway in Pseudomonas putida: kinetic and magnetic resonance studies of the cis,cis-muconate cycloisomerase catalyzed reaction. Biochemistry. 1983 Oct 25;22(22):5223–5230. doi: 10.1021/bi00291a024. [DOI] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsko G. A., Kenyon G. L., Gerlt J. A., Ringe D., Kozarich J. W. On the origin of enzymatic species. Trends Biochem Sci. 1993 Oct;18(10):372–376. doi: 10.1016/0968-0004(93)90091-z. [DOI] [PubMed] [Google Scholar]
- Powlowski J. B., Ingebrand J., Dagley S. Enzymology of the beta-ketoadipate pathway in Trichosporon cutaneum. J Bacteriol. 1985 Sep;163(3):1136–1141. doi: 10.1128/jb.163.3.1136-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Burbee D. G., Dagley S. Catabolism of L-tyrosine in Trichosporon cutaneum. J Bacteriol. 1979 May;138(2):425–430. doi: 10.1128/jb.138.2.425-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. 1. Purification and physical properties of 3-carboxy-cis-cis-muconate cyclase from Aspergillus niger. Eur J Biochem. 1974 Oct 2;48(2):549–556. doi: 10.1111/j.1432-1033.1974.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. 2. Subunit structure of the 3-carboxy-cis-cis-muconate cyclase of Aspergillus niger. Eur J Biochem. 1974 Oct 2;48(2):557–562. doi: 10.1111/j.1432-1033.1974.tb03797.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. Kinetic properties and mechanism of 3-carboxy-cis,cis-muconate cyclase from Aspergillus niger. Eur J Biochem. 1975 Aug 1;56(1):193–204. doi: 10.1111/j.1432-1033.1975.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi: conversion of beta-carboxymuconolactone into 3-oxoadipate in Aspergillus niger. Biochem J. 1970 Dec;120(4):28P–29P. doi: 10.1042/bj1200028pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou A. Y., Ransom S. C., Gerlt J. A., Buechter D. D., Babbitt P. C., Kenyon G. L. Mandelate pathway of Pseudomonas putida: sequence relationships involving mandelate racemase, (S)-mandelate dehydrogenase, and benzoylformate decarboxylase and expression of benzoylformate decarboxylase in Escherichia coli. Biochemistry. 1990 Oct 23;29(42):9856–9862. doi: 10.1021/bi00494a015. [DOI] [PubMed] [Google Scholar]
- Williams S. E., Woolridge E. M., Ransom S. C., Landro J. A., Babbitt P. C., Kozarich J. W. 3-Carboxy-cis,cis-muconate lactonizing enzyme from Pseudomonas putida is homologous to the class II fumarase family: a new reaction in the evolution of a mechanistic motif. Biochemistry. 1992 Oct 13;31(40):9768–9776. doi: 10.1021/bi00155a033. [DOI] [PubMed] [Google Scholar]
- Woods S. A., Schwartzbach S. D., Guest J. R. Two biochemically distinct classes of fumarase in Escherichia coli. Biochim Biophys Acta. 1988 Apr 28;954(1):14–26. doi: 10.1016/0167-4838(88)90050-7. [DOI] [PubMed] [Google Scholar]