Abstract
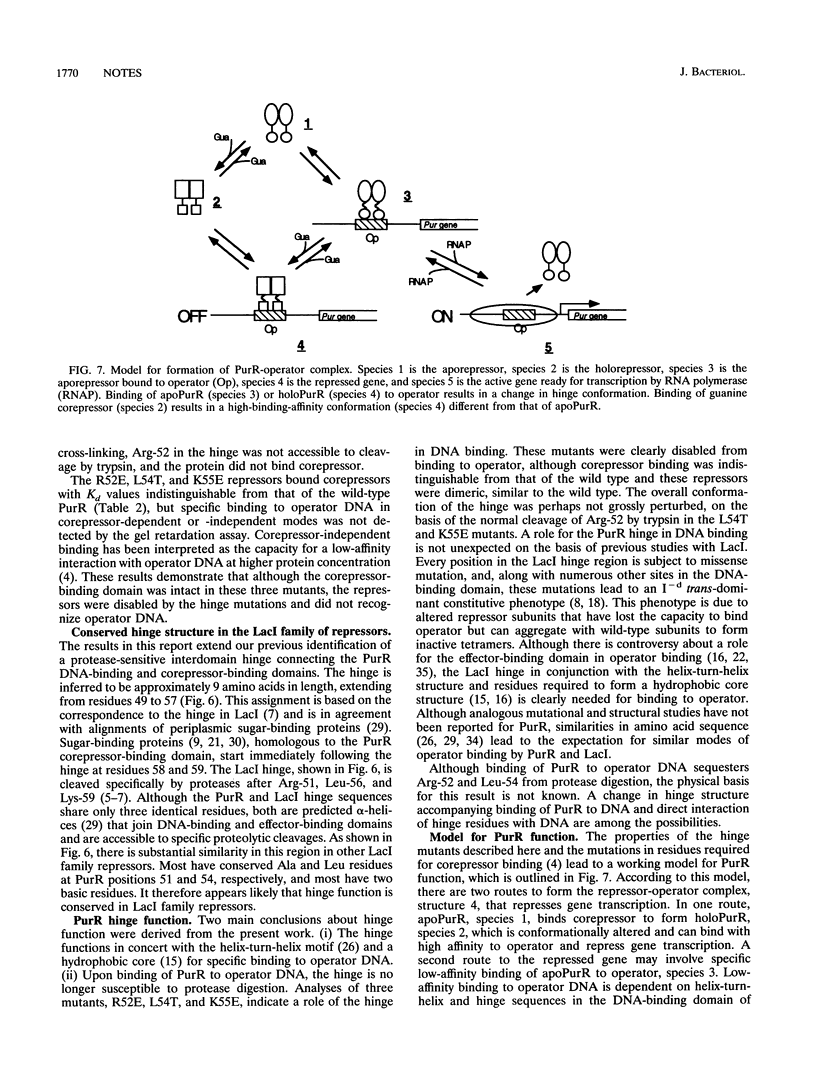
A protease-hypersensitive hinge sequence in Escherichia coli purine repressor (PurR) connects an N-terminal DNA-binding domain with a contiguous corepressor-binding domain. Binding of one molecule of dimeric repressor to operator DNA protects the hinge against proteolytic cleavage. Mutations in the hinge region impair repressor function in vivo. Several nonfunctional hinge mutants were defective in low-affinity binding to operator DNA in the absence of corepressor as well as in high-affinity corepressor-dependent binding to operator DNA, although binding of corepressor was similar to binding of the wild-type repressor. These results establish a role for the hinge region in operator binding and lead to a proposal for two routes to form the holoPurR-operator complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowie J. U., Lüthy R., Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991 Jul 12;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Zalkin H. Regulation of Escherichia coli pyrC by the purine regulon repressor protein. J Bacteriol. 1990 Jun;172(6):3201–3207. doi: 10.1128/jb.172.6.3201-3207.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. Y., Zalkin H. Structural characterization and corepressor binding of the Escherichia coli purine repressor. J Bacteriol. 1992 Oct;174(19):6207–6214. doi: 10.1128/jb.174.19.6207-6214.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files J. G., Weber K. Limited proteolytic digestion of lac repressor by trypsin. Chemical nature of the resulting trypsin-resistant core. J Biol Chem. 1976 Jun 10;251(11):3386–3391. [PubMed] [Google Scholar]
- Geisler N., Weber K. Escherichia coli lactose repressor: isolation of two different homogeneous headpieces and the existence of a hinge region between residues 50 and 60 in the repressor molecule. FEBS Lett. 1978 Mar 15;87(2):215–218. doi: 10.1016/0014-5793(78)80335-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of amino-terminal fragment of lactose repressor necessary for DNA binding. Biochemistry. 1977 Mar 8;16(5):938–943. doi: 10.1021/bi00624a020. [DOI] [PubMed] [Google Scholar]
- Gordon A. J., Burns P. A., Fix D. F., Yatagai F., Allen F. L., Horsfall M. J., Halliday J. A., Gray J., Bernelot-Moens C., Glickman B. W. Missense mutation in the lacI gene of Escherichia coli. Inferences on the structure of the repressor protein. J Mol Biol. 1988 Mar 20;200(2):239–251. doi: 10.1016/0022-2836(88)90237-9. [DOI] [PubMed] [Google Scholar]
- Groarke J. M., Mahoney W. C., Hope J. N., Furlong C. E., Robb F. T., Zalkin H., Hermodson M. A. The amino acid sequence of D-ribose-binding protein from Escherichia coli K12. J Biol Chem. 1983 Nov 10;258(21):12952–12956. [PubMed] [Google Scholar]
- He B., Choi K. Y., Zalkin H. Regulation of Escherichia coli glnB, prsA, and speA by the purine repressor. J Bacteriol. 1993 Jun;175(11):3598–3606. doi: 10.1128/jb.175.11.3598-3606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Shiau A., Choi K. Y., Zalkin H., Smith J. M. Genes of the Escherichia coli pur regulon are negatively controlled by a repressor-operator interaction. J Bacteriol. 1990 Aug;172(8):4555–4562. doi: 10.1128/jb.172.8.4555-4562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Zalkin H. Regulation of Escherichia coli purA by purine repressor, one component of a dual control mechanism. J Bacteriol. 1994 Feb;176(4):1009–1013. doi: 10.1128/jb.176.4.1009-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Zalkin H. Repression of Escherichia coli purB is by a transcriptional roadblock mechanism. J Bacteriol. 1992 Nov;174(22):7121–7127. doi: 10.1128/jb.174.22.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein R., Zuiderweg E. R., Scheek R. M., Boelens R., van Gunsteren W. F. A protein structure from nuclear magnetic resonance data. lac repressor headpiece. J Mol Biol. 1985 Mar 5;182(1):179–182. doi: 10.1016/0022-2836(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Khoury A. M., Nick H. S., Lu P. In vivo interaction of Escherichia coli lac repressor N-terminal fragments with the lac operator. J Mol Biol. 1991 Jun 20;219(4):623–634. doi: 10.1016/0022-2836(91)90659-t. [DOI] [PubMed] [Google Scholar]
- Kilstrup M., Meng L. M., Neuhard J., Nygaard P. Genetic evidence for a repressor of synthesis of cytosine deaminase and purine biosynthesis enzymes in Escherichia coli. J Bacteriol. 1989 Apr;171(4):2124–2127. doi: 10.1128/jb.171.4.2124-2127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleina L. G., Miller J. H. Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol. 1990 Mar 20;212(2):295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hogg R. W., Hermodson M. A. The amino acid sequence of the D-galactose-binding protein from Escherichia coli B/r. J Biol Chem. 1981 May 10;256(9):4350–4356. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Matthews K. S. Tryptic core protein of lactose repressor binds operator DNA. J Biol Chem. 1979 May 10;254(9):3348–3353. [PubMed] [Google Scholar]
- Meng L. M., Kilstrup M., Nygaard P. Autoregulation of PurR repressor synthesis and involvement of purR in the regulation of purB, purC, purL, purMN and guaBA expression in Escherichia coli. Eur J Biochem. 1990 Jan 26;187(2):373–379. doi: 10.1111/j.1432-1033.1990.tb15314.x. [DOI] [PubMed] [Google Scholar]
- Meng L. M., Nygaard P. Identification of hypoxanthine and guanine as the co-repressors for the purine regulon genes of Escherichia coli. Mol Microbiol. 1990 Dec;4(12):2187–2192. doi: 10.1111/j.1365-2958.1990.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Mowbray S. L., Cole L. B. 1.7 A X-ray structure of the periplasmic ribose receptor from Escherichia coli. J Mol Biol. 1992 May 5;225(1):155–175. doi: 10.1016/0022-2836(92)91033-l. [DOI] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Autoregulation of Escherichia coli purR requires two control sites downstream of the promoter. J Bacteriol. 1990 Oct;172(10):5758–5766. doi: 10.1128/jb.172.10.5758-5766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J Biol Chem. 1988 Dec 25;263(36):19653–19661. [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Purification of the Escherichia coli purine regulon repressor and identification of corepressors. J Bacteriol. 1990 Oct;172(10):5637–5642. doi: 10.1128/jb.172.10.5637-5642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M. A., Macdonald J. R., Björkman J., Mowbray S. L., Brennan R. G. Structural analysis of the purine repressor, an Escherichia coli DNA-binding protein. J Biol Chem. 1993 Jun 15;268(17):12282–12288. [PubMed] [Google Scholar]
- Scripture J. B., Voelker C., Miller S., O'Donnell R. T., Polgar L., Rade J., Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Nucleotide sequence and analysis of gene products. J Mol Biol. 1987 Sep 5;197(1):37–46. doi: 10.1016/0022-2836(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Steiert J. G., Rolfes R. J., Zalkin H., Stauffer G. V. Regulation of the Escherichia coli glyA gene by the purR gene product. J Bacteriol. 1990 Jul;172(7):3799–3803. doi: 10.1128/jb.172.7.3799-3803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weickert M. J., Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992 Aug 5;267(22):15869–15874. [PubMed] [Google Scholar]
- Wilson H. R., Turnbough C. L., Jr Role of the purine repressor in the regulation of pyrimidine gene expression in Escherichia coli K-12. J Bacteriol. 1990 Jun;172(6):3208–3213. doi: 10.1128/jb.172.6.3208-3213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Müller-Hill B. Sequence of galR gene indicates a common evolutionary origin of lac and gal repressor in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2427–2431. doi: 10.1073/pnas.79.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]