Abstract
Toll-like receptor 2 (TLR2) and TLR4 signaling may induce differential secretion of T helper 1 (Th1) and Th2 cytokines, potentially influencing the development of autoimmune or atopic diseases. To date, the influence of the type of stimulus, timing, and dose of TLR2 and TLR4 ligands on cytokine secretion has not been well established. We tested whether the innate stimuli peptidoglycan (Ppg, TLR2 agonist) and lipid A (LpA, TLR4 agonist) differentially affect the secretion of interleukin-13 (IL-13) (Th2) and interferon-γ (IFN-γ) (Th1). Further, we examined the influence of the maturity of the immune system, species, dose, and timing of stimuli in human cord and adult peripheral blood mononuclear cells (PBMC) and murine cells in vitro and in vivo. Stimulation with Ppg induced the secretion of both IL-13 and IFN-γ, influenced by time and dose in neonates, adults, and mice. In contrast, stimulation with LpA induced primarily time-independent and dose-independent production of IFN-γ. Pulmonary administration of Ppg in vivo in mice resulted in secretion of IL-13, whereas administration of LpA resulted in secretion of IFN-γ in bronchoalveolar lavage (BAL). Therefore, TLR2 and TLR4 stimuli differentially influence IL-13 and IFN-γ secretion in neonates, adults, and mice, supporting a critical role for innate stimuli in the modulation of cytokine responses.
INTRODUCTION
Conserved throughout evolution, mammalian toll-like receptors (TLR) participate in innate immune responses to microbial pathogens. TLR proteins mediate cellular activation, including the expression of cytokines induced by a variety of bacterial products. The ligands for TLR include the cell wall component of gram-positive bacteria, peptidoglycan (Ppg), which is predominantly recognized by TLR2, and of gram-negative bacteria, lipopolysaccharide (LPS), and its bioactive component lipid A (LpA), which are recognized by TLR4.(1–9) TLRs are characterized by an extracellular leucin-rich domain that shows considerable diversity. Although the TLRs for which an agonist has been identified all appear to activate similar signaling pathways, including NF-κB, p38 mitogen-activated protein kinase (MAPK), stress-activated protein (SAP)/JNK, and MAPK (Erk1/2) kinases,(2,10) it is not clear whether different TLRs differ in their ultimate function: the activation of innate immune responses. Despite a high sequence homology, the cytoplasmic tails of different TLRs are not functionally equivalent, suggesting that different signals may emanate from distinct receptors. Also, accessory molecules, such as myeloid differentiation-2 (MD-2) for TLR4, and different adaptor molecules may play a role in the induction of distinct cytokine patterns.(11–13) Depending on the type of TLR ligand, in addition to timing and dose of stimulus, different cell types, such as dendritic cells (DC), mature and prime T lymphocytes to specific T helper 1 (Th1) or Th2 responses.(14,15) Although TLR2 and TLR4 ligands induce primarily Th1 immune responses,(16) TLR2 signaling can also promote a Th2-favored cytokine pattern.(5)
The type and timing of innate exposure are important in the evolution of several immune-mediated diseases. For example, epidemiologic studies have shown that exposure to endotoxin (LPS) may protect against the development of Th2-mediated diseases in childhood, such as asthma and allergies. Exposure to the TLR4 ligand endotoxin early in life may be critical in the modulation of allergic diseases.(17–22) Whether other microbes, such as gram-positive bacteria (TLR2 ligands), may influence the development of the innate immune system remains undefined. Also, whether these immune responses may differ in early life (neonates) and later in life (adults) is unknown.
We tested whether TLR2 and TLR4 stimulation may induce differential cytokine secretion and further assessed the effects in relationship to dose and timing of the stimuli. We examined interleukin-13 (IL-13) (Th2) and interferon-γ (IFN-γ) (Th1) secretion following TLR2 (Ppg) and TLR4 (LpA or LPS) stimulation of human (neonatal and adult blood) mononuclear cells. Further, we evaluated Ppg and LpA-induced murine secretion of IL-13 and IFN-γ by spleen cells in vitro and in bronchoalveolar lavage (BAL) fluid following pulmonary administration of Ppg and LpA in vivo. In addition, we examined the effects of administration of LpA in a murine model of allergic inflammation on allergen-induced airway hyperreactivity (AHR). These approaches may offer the possibility of investigating future mechanisms of innate stimuli in human and murine models.
MATERIALS AND METHODS
Analysis of human samples
Characteristics of human population
Cord blood samples (n = 30) were obtained from a subgroup of a Boston area pregnancy/birth cohort(23) based on technical availability. Mothers in this subgroup were healthy, as determined by questionnaire and medical records. Exclusion criteria included maternal fever, maternal diseases, medications, and delivery by cesarean section. Healthy adult volunteers (n = 7) between the ages of 27 and 50 years (median 32 years) were recruited at the Brigham and Women’s Hospital and consented to phlebotomy. Approval for both studies was obtained from the Human Subjects Review Committee of Brigham and Women’s Hospital, Boston.
Isolation of human mononuclear cells and cell preparation
Cord blood samples were collected from the umbilical vein after delivery. Blood samples from adults were collected by withdrawing blood from a peripheral vein and were processed as previously described.(24) Samples were placed in heparinized tubes and processed within 24 h. Cord blood and peripheral blood mononuclear cells (CBMC, PBMC) were isolated by density-gradient centrifugation with Ficoll-Hypaque Plus (Pharmacia, Uppsala, Sweden) after dilution in phosphate-buffered saline (PBS), (Sigma Aldrich, St. Louis, MO). Cells were washed in RPMI 1640 and diluted in 10% human serum (Biowhittaker, Walkersville, MD) to a concentration of 5 × 106 cells/ml. Supernatants from cell cultures were harvested at 16, 24, or 72 h after stimulation with Ppg (0.1, 1, 10, 100 μg/ml) (Staphylococcus aureus, Sigma Aldrich) and LpA (0.01, 0.1, 1, 100 μg/ml) (Salmonella minnesota Re-595, Sigma Aldrich), and with phytohemagglutinin (PHA) (5 μg/ml) (Sigma Aldrich) as positive control, and they were compared with unstimulated samples. Endotoxin concentrations in Ppg and PHA, measured by limulus assay, were very low (<0.01 EU/ml = 0.002 ng/ml) and did not significantly change lymphocyte proliferation or cytokine secretion in CBMC. By testing the functional ability of CBMC with different doses of LPS and active components, such as LpA (starting at 0.01 ng to 100 ng/ml), we detected increased lymphocyte proliferation with doses of LpA > 1 ng/ml.
Cytokine secretion
Supernatants were aliquoted in duplicate into 96-well plates (50 μl/well) precoated with cytokine-specific antibody. Optical density was measured at 450 nm. The cytokines IL-13 and IFN-γ were measured with commercially available human ELISA kits (Endogen, Rockford, IL) according to the manufacturer’s instructions. The lower limits of detection were 7.0 pg/ml for IL-13 and 2.0 pg/ml for IFN-γ.
Lymphocyte proliferation
For the lymphocyte proliferation assay, human CBMC and PBMC (0.5 × 106 cells/well) were cultured in quadruplicate in 96-well plates (Corning, New York, NY) for 3 days and pulsed with 1 μCi 3H-thymidine for an additional 8 h. Cultures were held at 37°C in a humidified 5% CO2 incubation chamber. Cells were harvested with a Tomcat Mach II harvester (Wallac, Turku, Finland) onto filter plates, which were read with a β-counter. Proliferation was quantified by stimulation index (SI), which is calculated as the ratio of mean counts per minute (cpm) of stimulated over unstimulated replicates.
Analysis of murine samples
Mice
Six–eight-week-old inbred BALB/c (Jackson Laboratory, Bar Harbor, ME), TLR2 knockout (KO) (R. Medzhitov, Yale University, New Haven, CT), and WT c57Bl/6 female and male mice were maintained according to the guidelines of the Committee on Animals of the Harvard Medical School and the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources National Research Council.
In vitro protocol
Spleen cells from mice (n = 5 per group) were isolated,(25) washed with RPMI 1640, and suspended in RPMI 1640 with 10% fetal bovine serum (FBS) and antibiotics (100 μg/ml penicillin, 100 U/ml streptomycin, 2 mM L-glutamine) to a concentration of 5 × 106 cells/ml. Cell culture supernatants were harvested after stimulation for 22 and 44 h in vitro with Ppg (0.1, 1, 10, 100 μg/ml), LPS (0.1, 1, 10 μg/ml), and LpA (0.1, 1, 10, 100 μg/ml) and compared with supernatants of cells stimulated with PHA (5 μg/ml) as a positive control and with unstimulated samples.
In vivo protocol
Each mouse received i.p. anesthesia with ketamine/xylazine and underwent intratracheal (i.t.) administration of Ppg (75 μg), LpA (75 μg), or PBS (0.2 ml of 0.5× PBS). Each mouse underwent BAL as previously described.(26,27) BAL cells were pelleted, and the supernatant was stored at −80°C. Mice were sacrificed by cardiac puncture at 18 or 42 h after stimulation (n = 6 mice per group).
In a subset of experiments, mice underwent a protocol of allergic inflammation consisting of sensitization and challenge with the allergen ovalbumin (OVA) as previously described.(26,27) Briefly, mice were sensitized by i.p. injection with 10μg chicken OVA (or PBS) and 1 mg Al(OH)3 on days 0 and 7 and challenged with 6% OVA (or PBS) for 20 min/day on days 14–20 via an ultrasonic nebulizer (model 5000, DeVilbiss, Somerset, PA). In addition, LpA was administered i.t. to OVA (or PBS) sensitized and challenged mice 1 day before sensitization. AHR before and after a 2-min metacholine nebulization (100 mg/ml) was determined 24 h after the final aerosol challenge using whole-body plethysmography as previously described.(25–27) The whole-body plethysmography system measures changes in box pressure during expiration and inspiration and generates a value called enhance pause (Penh = PEP/PIPx (Te-Tr/Tr)) directly correlating with airway resistance.
Murine cytokine secretion
Cytokine concentrations were measured with ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). In brief, spleen cell samples or BAL samples were aliquoted in duplicate into 96-well plates (50 μl/well) precoated with cytokine-specific antibody. Optical density (OD) was measured at 450 nm. Cytokine concentrations were determined by comparison with known standards. The lower limits of detection were 1.5 pg/ml for IL-13 and 2.0 pg/ml for IFN-γ.
Murine lymphocyte proliferation
The lymphocyte proliferation assay is identical to the human protocol described earlier. In brief, spleen cells were aliquoted into 96-well plates and stimulated for 44 h at 37°C in 5% CO2 in the presence and absence of 10 μg/ml Ppg, 100 μg/ml LpA, and 10 μg/ml LPS.
Statistical analysis
Data analysis was performed with Sigma Stat software. For continuous variables, either the t-test or Wilcoxon/Kruskal Wallis rank sum test was performed, depending on the distribution of the data. Statistically significant differences for comparison of several groups were determined by one-way ANOVA, followed by a comparison of groups with the Tukey-Kramer analysis. Data are reported as mean ± SEM. Statistical significance was defined by p < 0.05.
RESULTS
TLR2 and TLR4 stimulation differentially modulate neonatal IL-13 and IFN-γ cytokine secretion
The specificity of TLR ligands was determined by administering LpA and Ppg to wild-type (WT) and TLR2 KO mice (Fig. 1). LpA stimulation induced lymphocyte proliferation (SI) in WT and TLR2 KO mice, whereas Ppg induced proliferation in WT but not in TLR2 KO mice.
FIG. 1.
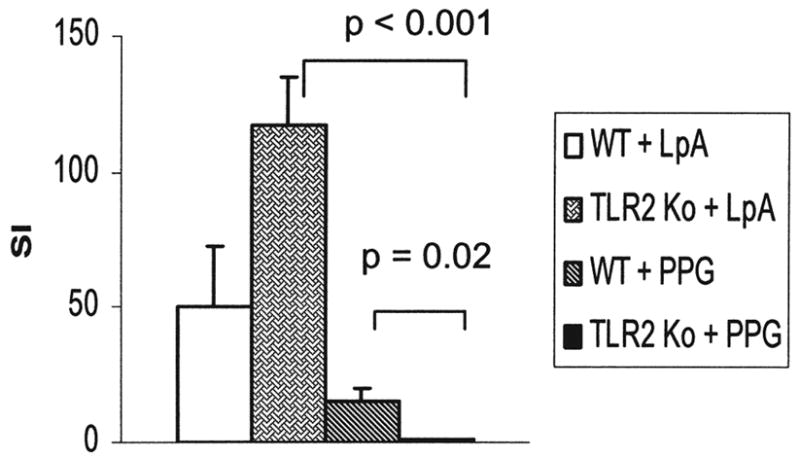
Lymphocyte proliferation following innate stimulation in WT and TLR2 KO mice. Stimulation with LpA (TLR4) induced lymphocyte proliferation in WT as well as TLR2 KO mice. In contrast, stimulation with Ppg (TLR2) induced lymphocyte proliferation only in WT but not in TLR2 KO mice. Lymphocyte proliferation was determined as stimulation index (SI) following stimulation of murine spleen cells with LpA and Ppg (both 100 μg/ml) for 72 h by 3H-thymidine uptake, as described in Materials and Methods (n = 5 mice per group).
To analyze the effect of innate stimuli on immune responses of CBMC, we measured the secretion of IL-13 (a Th2 cytokine) and IFN-γ (a Th1 cytokine) after stimulation with the TLR2 ligand Ppg or the TLR4 ligand LpA for 72 h. We chose to examine CBMC to assess the interaction of T cells with antigen-presenting cells (APCs). In cord blood, the production of IL-13 was highest in response to stimulation with Ppg, intermediate in response to stimulation with LpA, and low in unstimulated cells (Fig. 2A). IFN-γ secretion was increased significantly following stimulation with Ppg and LpA compared with secretion in un-stimulated cells (p = 0.002 and p = 0.001) (Fig. 2B). However, there was no significant difference between Ppg-induced and LpA-induced IFN-γ secretion. Proliferation of CBMC was intact, indicating viability of the cells (Fig. 2C). Proliferation induced by Ppg and LpA was higher than was proliferation in un-stimulated cells (p < 0.001), whereas the proliferative responses to Ppg and LpA were not significantly different (p = 0.09) (Fig. 2C). PHA, used as a positive control, induced proliferation of CBMC with an SI of 33 ± 8 (data not shown).
FIG. 2.
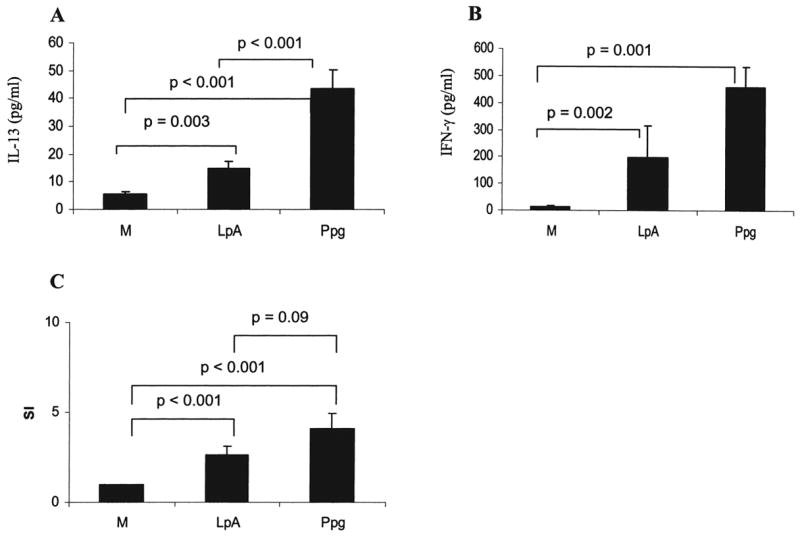
CBMC IL-13 and IFN-γ secretion following innate stimulation. (A) Secretion of IL-13 by CBMC following stimulation with Ppg was increased compared with secretion following LpA stimulation (p < 0.001). Both stimuli induced higher levels of IL-13 secretion than in unstimulated cells (M) (p = 0.003 and p < 0.001). (B) CBMC stimulated with LpA and Ppg showed higher levels of IFN-γ secretion than in un-stimulated CBMC (p = 0.002 and p = 0.001). (C) Lymphocyte proliferation was intact at 72 h after stimulation with LpA and Ppg. (A and B) Cytokine concentrations from supernatants of CBMC harvested 72 h after stimulation with LpA (0.1 μg/ml) and Ppg (10 μg/ml) were measured with ELISA. (C) Lymphocyte proliferation was determined following stimulation with the indicated doses of LpA and Ppg for 72 h by 3H-thymidine uptake, as described in Materials and Methods (n = 30 for M and Ppg, n = 15 for LpA).
Secretion of IL-13 and IFN-γ induced by TLR2 and TLR4 stimulation is similar in adult and neonatal mononuclear cells
Because Ppg seems to influence the responses of CBMC toward a greater IL-13 response than does LpA, we tested whether this cytokine pattern was retained in PBMC of healthy mature adults. Interestingly, in PBMC, secretion of IL-13 and IFN-γ after stimulation with LpA and Ppg was comparable to that in CBMC (Fig. 3). These data indicate that similar TLR2-induced and TLR4-induced cytokine secretions as seen in CBMC are present in healthy adult PBMC.
FIG. 3.
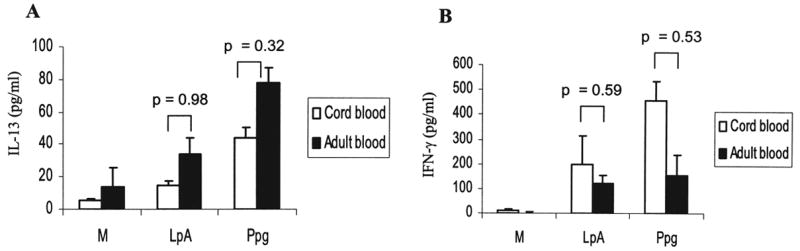
Comparison of IL-13 and IFN-γ secretion in CBMC and PBMC following administration of different innate stimuli. Secretion of (A) IL-13 and (B) IFN-γ in cord blood (n = 30 for M and Ppg, n = 15 for LpA) and in adults (n = 7) was similar after stimulation with LpA and Ppg. Cytokine concentrations in supernatants of PBMC harvested 72 h after stimulation with LpA (0.1 μg/ml) and Ppg (10 μg/ml) were measured using ELISA.
TLR2 stimulation induces secretion of both IL-13 and IFN-γ, whereas TLR4 stimulation induces primarily secretion of IFN-γ in murine spleen cells
We assessed whether the cytokine pattern observed in humans in response to Ppg and LpA was also found in the murine system. This may offer the possibility to examine mechanisms of innate immune responses in nonhuman subjects. Secretion of IL-13 and IFN-γ by spleen cells stimulated with Ppg was significantly increased in comparison to that induced by LpA and LPS and in unstimulated cells (p < 0.001) (Fig. 4A, B). In contrast, secretion of IL-13 induced by LpA and LPS showed no significant increase compared with secretion by unstimulated cells (Fig. 4A, B). Spleen cell proliferation was preserved, indicating cell viability (Fig. 4C). The control concanavalin A (ConA) was positive, with an SI of 49 ± 6 (data not shown). These results indicate that in the murine species, similar to humans, TLR2 stimulation induces secretion of IL-13 and IFN-γ, and TLR4 stimulation induces primarily IFN-γ secretion. In humans, however, TLR2-induced and TLR4-induced IFN-γ secretion is similar, whereas in mice, TLR2 induced higher levels of IFN-γ than did TLR4.
FIG. 4.
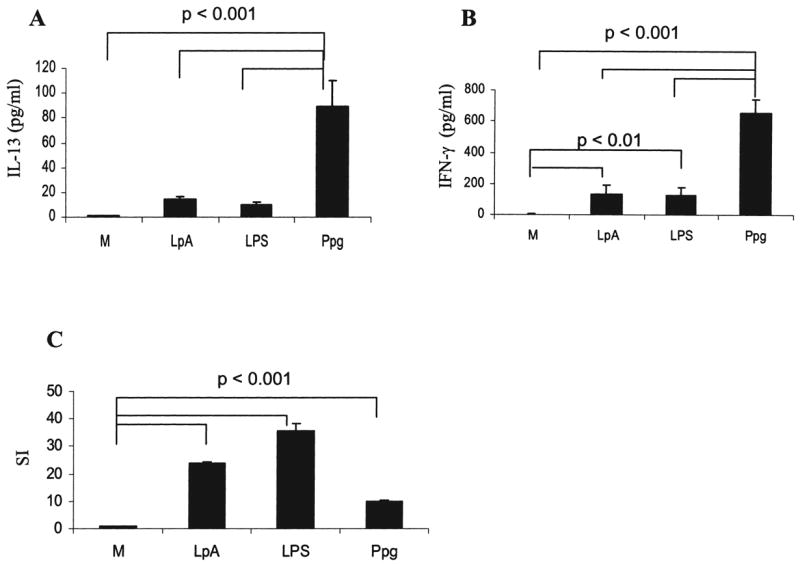
Secretion of IL-13 and IFN-γ by isolated murine spleen cells. Secretion of (A) IL-13 and (B) IFN-γ by isolated murine spleen cells was higher after stimulation with Ppg compared with cells stimulated with LpA or LPS and unstimulated cells (M) (p < 0.001). IFN-γ secretion after stimulation with LpA and LPS was increased compared with secretion by un-stimulated cells (M) (p < 0.01). (C) Lymphocyte proliferation was intact following stimulation with Ppg, LpA, and LPS. (A, B, and C) Cytokine concentrations from supernatants of spleen cells harvested at 44 h after stimulation with 10 μg/ml Ppg, 100 μg/ml LpA, and 10 μg/ml LPS were measured using ELISA. Lymphocyte proliferation was determined following stimulation with the indicated doses of Ppg, LpA, and LPS for 44 h by 3H-thymidine uptake, as described in Materials and Methods (n = 6 mice per group).
Secretion of IL-13 and IFN-γ following stimulation of TLR2 is influenced by dose and timing in humans and mice
Levels of IL-13 induced by Ppg in human PBMC were higher than those induced by LpA (Fig. 5). This increase was highest with the Ppg dose of 10 μg/ml (Fig. 5A) and after 72 h of stimulation of PBMC (Fig. 5C). In contrast, LpA-induced IL-13 secretion was present but did not change in response to different doses or timing (Fig. 5B, C). Secretion of IFN-γ was undetectable in unstimulated PBMC (data not shown). Stimulation with the TLR2 agonist Ppg induced higher levels of IFN-γ secretion in a dose-dependent and time-dependent manner compared with the TLR4 agonist LpA (data not shown).
FIG. 5.
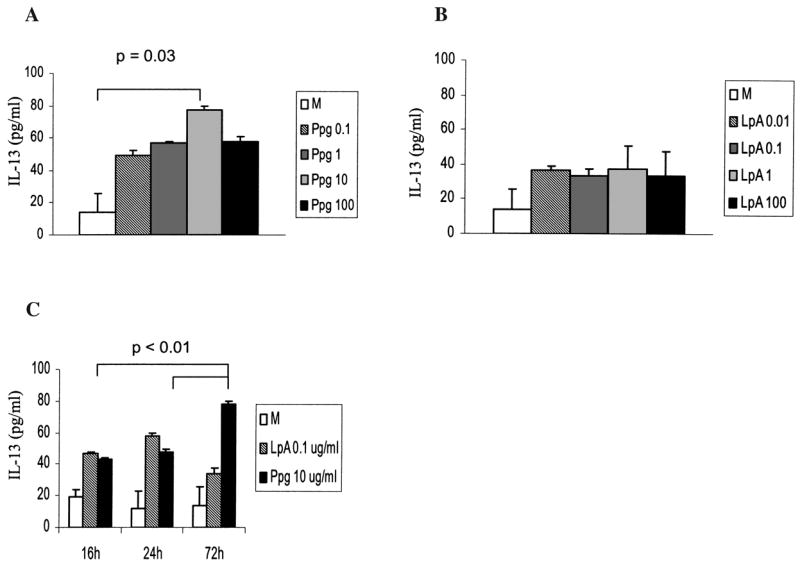
Dose-dependent and time-dependent effects on secretion of IL-13 following stimulation with Ppg and LpA in human adult PBMC. (A) IL-13 secretion induced by Ppg stimulation was dose dependent and significantly higher with 10 μg/ml Ppg than in un-stimulated cells (p = 0.03). (B) IL-13 secretion induced by LpA stimulation was independent of dose. (C) IL-13 secretion of un-stimulated cells (M) and following LpA stimulation was independent of time. IL-13 secretion was higher after 72 h of stimulation with 10 μg/ml Ppg than after 24 and 16 h of stimulation (p < 0.01). (A, B, and C) Cytokine concentrations from supernatants of adult PBMCs were harvested at the indicated times after stimulation with the indicated doses (μg/ml) of Ppg and LpA and measured using ELISA (n = 7).
In murine spleen cells, Ppg induced higher levels of IL-13 and IFN-γ secretion with increasing doses of Ppg and higher levels at 44 h than at 22 h (Fig. 6A, D, E, H). In contrast, secretion of IL-13 induced by LpA and LPS was very low and did not reach statistical significance at any dose (Fig. 6B, C, D). Also, LpA-induced and LPS-induced IFN-γ secretion was independent of dose and timing (Fig. 6F, G, H); levels of IL-13 induced by LpA and LPS were significantly lower than those following Ppg stimulation (Fig. 4). In this study of human and murine cells, stimulation with LpA or LPS caused a predominant Th1 (IFN-γ) pattern of cytokine secretion independent of dose and timing. In contrast, Ppg showed a pronounced increase in both IL-13 and IFN-γ secretion, which was influenced by dose and time, resulting in a cytokine pattern skewed toward IL-13 as compared with the pattern for TLR4 agonists, which induce primarily secretion of IFN-γ.
FIG. 6.
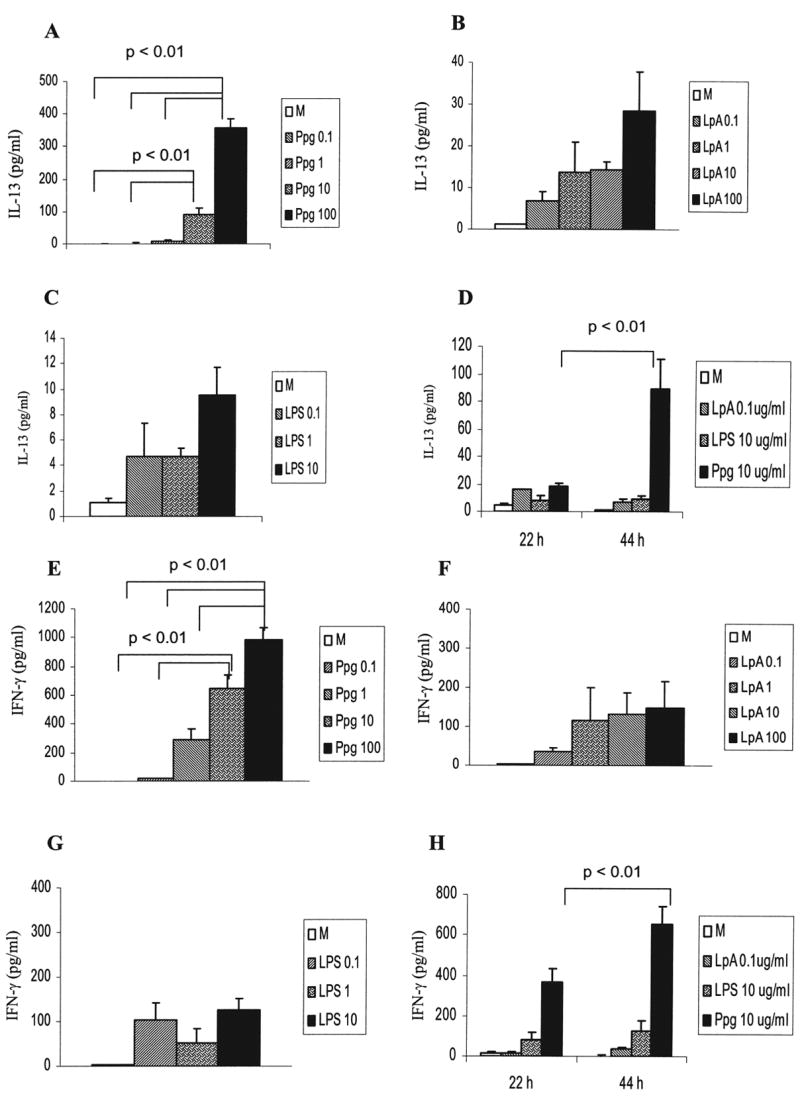
Dose-dependent and time-dependent effects on secretion of IL-13 and IFN-γ following stimulation with Ppg, LpA, and LPS in murine spleen cells. (A and E) Higher doses of Ppg resulted in higher levels of IL-13 and IFN-γ secretion (p < 0.01). (B, C, F, and G) IL-13 and IFN-γ secretion induced by LpA and LPS was independent of dose. (D and H) IL-13 and IFN-γ secretion increased from 22 to 44 h after stimulation with Ppg (p < 0.01) and was similar after stimulation with LpA and LPS. (A, B, C, D, E, F, G, and H) Cytokine concentrations from supernatants of murine spleen cells harvested at 44 h (A, B, C, E, F, and G) or at 22 h and 44 h after stimulation with the indicated doses (μg/ml) of Ppg, LpA and LPS (D and H) were measured with ELISA (n = 6 mice per group).
Intrapulmonary Ppg administration induces primarily IL-13 secretion in BAL, whereas LpA preferentially induces IFN-γ secretion in vivo
Applying of Ppg and LpA i.t., we tested whether TLR2 and TLR4 agonists exerted differential patterns of cytokine secretion in a murine model in vivo. Whereas pulmonary stimulation in vivo resulted in higher levels of IL-13 secretion in BAL in response to Ppg than to LpA and PBS at 42 h (p < 0.01) (Fig. 7A), IFN-γ secretion was significantly higher with LpA than with Ppg and PBS at the same time point (p < 0.01) (Fig. 7B). Time-dependent increases were seen in Ppg-induced secretion of IL-13 and LpA-induced secretion of IFN-γ (p < 0.01). Taken together, our results show that Ppg induces similar responses in humans and the murine species. TLR2 stimulation triggers the expression of significantly higher levels of IL-13 by mononuclear cells in both humans and mice and, only in mice, significantly higher secretion of IFN-γ compared with stimulation with TLR4. In humans, secretion of IFN-γ induced by TLR2 is similar to that induced by TLR4. These effects are dose and time dependent. In contrast, in both humans and the murine species, a TLR4 agonist induces primarily the secretion of IFN-γ (Th1) independently of dose and time.
FIG. 7.
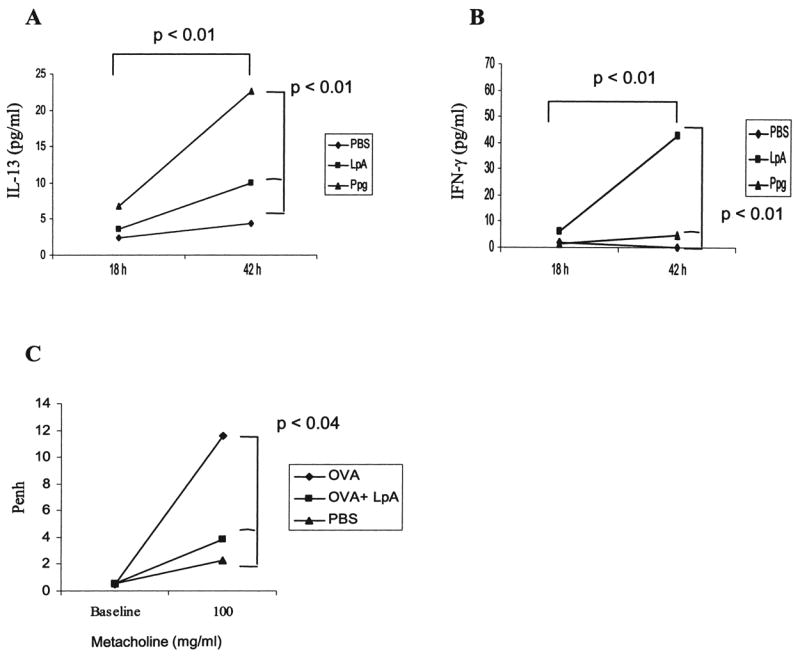
Pulmonary stimulation with LpA and Ppg in vivo. (A and B) Intratracheal (i.t.) stimulation with Ppg induced higher levels of IL-13 in BAL fluid in mice compared with stimulation with LpA and PBS, whereas IFN-γ secretion was higher following stimulation with LpA compared with Ppg and PBS (p < 0.01). Mice underwent i.t. administration of Ppg (75 μg), LpA (75 μg), or PBS (0.2 ml of 0.5× PBS), and BAL was performed 18 or 42 h later, as previously described.(26,27) Cytokine concentrations from supernatants of BAL cells were measured with ELISA as described in Materials and Methods (n = 6 mice per group). (C) AHR (Penh) was increased in OVA-sensitized and challenged mice compared with PBS mice. OVA mice treated with LpA i.t. showed significantly decreased Penh compared with OVA mice. AHR was determined by Penh measured at baseline and after metacholine challenge (100 mg/ml) in a murine model of allergic sensitization and challenged with OVA, as described in Materials and Methods (n = 6 mice per group).
In a murine model of allergic inflammation,(26,27) allergen OVA sensitization and challenge resulted in increased AHR, shown as Penh, in comparison to saline (PBS)-exposed mice (Fig. 7C). Local pulmonary administration of LpA before OVA sensitization reduced allergen-induced AHR, demonstrating an in vivo relevance of the TLR4 agonist.
DISCUSSION
We demonstrate that stimulation of TLR2 with Ppg induces secretion of both IL-13 and IFN-γ in humans and mice. These effects are dose and time dependent. In contrast, stimulation of TLR4 with LpA induces predominantly the secretion of IFN-γ in both humans and mice and IL-13 secretion, albeit at low levels. In humans, this cytokine pattern is observed in both neonates and adults.
Compared with TLR4, TLR2 stimulation induces predominantly IL-13 secretion. Recent studies have focused primarily on the analysis of proinflammatory or Th1 cytokines following TLR2 activation.(28–30) TLR2 activation of DCs has also resulted in a Th2-favored cytokine milieu,(5,14,31) and only one recent study analyzed the secretion of IL-13.(32) Studies on isolated mast cells support our finding that activation of TLR2 increases the secretion of IL-13.(29,30) We analyzed CBMC and PBMC to assess the interaction of APCs, DCs, and T cells following TLR2 and TLR4 stimulation. TLR4 activation induces secretion of IFN-γ in accordance with the findings of previous studies.(3,5,33) Although the signaling pathways of TLR2 and TLR4 converge to the same adaptor protein myeloid differentiation factor 88 (MyD88), their cytokine production patterns after stimulation are different. Whereas the mechanism of TLR4-induced IFN-γ secretion via IL-12 has been described,(34–38) the pathway of TLR2-induced IL-13 induction is less clear, potentially involving a more Th2 cytokine pattern via IL-12 suppression.(32)
Analysis of human neonatal and adult mononuclear cells revealed that Ppg induced secretion of IL-13 and IFN-γ and LpA preferentially induced secretion of IFN-γ. One interpretation of the conservation of cytokine patterns in humans is the maintenance of an early priming of mononuclear cells in adult life. Cytokine secretion by CBMC in the early immune system has been described as predominantly Th2.(39,40) The impairment in the capacity to induce Th1 immune responses may be attributed in part to a deficiency in APC function.(41) Nevertheless, unstimulated CBMC from healthy neonates in our study secreted low levels of both IL-13 and IFN-γ. In contrast to our findings of similar secretion of IFN-γ in neonates and adults following innate stimulation, another study reported lower levels of IFN-γ production in neonates than in adults.(42) One potential explanation for these different results is technical differences, including exposure to distinct microbes for a different duration. Neonatal immune responses are assumed to be quite different from adult responses, but depending on the particular immune environment, neonatal responses may be more mature than previously thought.(43,44) Priming of neonatal mononuclear cells is influenced by a variety of factors, such as the intrauterine environment, postnatal exposure to microbial, parasitic, or allergic stimuli, and intervention via vaccination.(39,44–52) Our study provides evidence that postnatal exposure to different microbial stimuli can skew the neonatal immune system to a cytokine secretion toward IFN-γ or IL-13. Whether this has implications in the development of Th1 or Th2-associated diseases remains to be determined.
In addition to age, we investigated dose, timing of administration of TLR2 and TLR4 agonists, and species, which may be important factors in innate immune responses. Some studies described dose-dependent effects on primarily proinflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNF-α), following stimulation with LPS or Ppg.(53,54) We found that in humans (neonates and adults) and mice, Ppg-induced secretion of IL-13 and IFN-γ was influenced by dose and timing. In contrast, LpA-induced and LPS-induced cytokine secretion was not influenced by dose and time. In the murine system, in vitro stimulation of TLR2 induces secretion of IL-13 and IFN-γ, and stimulation of TLR4 induces predominantly secretion of IFN-γ, similar to humans. These findings are applicable to the pulmonary environment. Notably, our murine studies indicate that pulmonary administration of Ppg in vivo induces predominantly the production of IL-13, and LpA induces primarily IFN-γ secretion. In a murine model of allergic inflammation, the decrease in allergen-induced AHR following administration of LpA supports the physiologic relevance of the TLR4 agonist. These findings in the pulmonary microenvironment may be helpful for elucidating mechanisms of innate immune modulation. The importance of timing of environmental exposure is exemplified by the development of asthma and allergic diseases. For example, early life exposure to an endotoxin-rich farm environment may have a protective effect on the development of Th2-mediated disease, such as asthma and allergies at school age, although this is controversial.(18–20,55–59)
Together, our findings provide insight that distinct innate stimuli may induce different cytokine responses, depending on the dose and timing of exposure, in both humans and murine species. We have shown that in human and murine mononuclear cells, TLR2 and TLR4 agonists differentially induce IL-13 and IFN-γ secretion. Importantly, our results emphasize that neonatal predilection of the immune system can be influenced by TLR2 and TLR4 stimulation, and a similar pattern of IL-13 and IFN-γ secretion induced by TLR2 and TLR4 stimuli is also observed in adult life. Whether the development of such diseases as asthma or autoimmune diseases, established early in life, may be influenced by interaction with TLR2 and TLR4 agonists awaits further investigation.
Acknowledgments
We thank Thomas Mueller for the thoughtful critique of this paper. This work was supported by NIH grants HL 56723, HL 67684, IA 45007, AI 045007 (all to P.W.F.), HL 64925, HL 68041 (to M.W.G.), HD 34568 (to D.G., M.W.G.), AI/EHS 535786 (to D.G.), HL 61907 (to S.T.W.), and DFG 997/1-1 (to B.S.).
References
- 1.YOSHIMURA A, LIEN E, INGALLS RR, TUOMANEN E, DZIARSKI R, GOLENBOCK D. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 2.TAKEUCHI O, HOSHINO K, KAWAI T, SANJO H, TAKADA H, OGAWA T, TAKEDA K, AKIRA S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 3.SCHNARE M, BARTON GM, HOLT AC, TAKEDA K, AKIRA S, MEDZHITOV R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 4.SABROE I, JONES EC, USHER LR, WHYTE MK, DOWER SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 5.RE F, STROMINGER JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 6.McCURDY JD, OLYNYCH TJ, MAHER LH, MARSHALL JS. Cutting edge: distinct toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 7.MASUDA A, YOSHIKAI Y, AIBA K, MATSUGUCHI T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 8.MARTIN M, MICHALEK SM, KATZ J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AKIRA S, TAKEDA K, KAISHO T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 10.HEMMI H, TAKEUCHI O, KAWAI T, KAISHO T, SATO S, SANJO H, MATSUMOTO M, HOSHINO K, WAGNER H, TAKEDA K, AKIRA S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.RE F, STROMINGER JL. Separate functional domains of human MD-2 mediate toll-like receptor 4-binding and lipopolysaccharide responsiveness. J Immunol. 2003;171:5272–5276. doi: 10.4049/jimmunol.171.10.5272. [DOI] [PubMed] [Google Scholar]
- 12.GUILLOT L, MEDJANE S, LE-BARILLEC K, BALLOY V, DANEL C, CHIGNARD M, SI-TAHAR M. Response of human pulmonary epithelial cells to lipopolysaccharide involves toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 13.AKASHI S, SAITOH S, WAKABAYASHI Y, KIKUCHI T, TAKAMURA N, NAGAI Y, KUSUMOTO Y, FUKASE K, KUSUMOTO S, ADACHI Y, KOSUGI A, MIYAKE K. Lipopolysaccharide interaction with cell surface toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DE JONG EC, VIEIRA PL, KALINSKI P, SCHUITEMAKER JH, TANAKA Y, WIERENGA EA, YAZDANBAKHSH M, KAPSENBERG ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 15.EISENBARTH SC, PIGGOTT DA, HULEATT JW, VISINTIN I, HERRICK CA, BOTTOMLY K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KOPP E, MEDZHITOV R. Recognition of microbial infection by toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 17.BRAUN-FAHRLANDER C, LAUENER R. Farming and protective agents against allergy and asthma. Clin Exp Allergy. 2003;33:409–411. doi: 10.1046/j.1365-2222.2003.01650.x. [DOI] [PubMed] [Google Scholar]
- 18.RIEDLER J, BRAUN-FAHRLANDER C, EDER W, SCHREUER M, WASER M, MAISCH S, CARR D, SCHIERL R, NOWAK D, VON MUTIUS E. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 19.BRAUN-FAHRLANDER C, RIEDLER J, HERZ U, EDER W, WASER M, GRIZE L, MAISCH S, CARR D, GERLACH F, BUFE A, LAUENER RP, SCHIERL R, RENZ H, NOWAK D, VON MUTIUS E. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 20.VON MUTIUS E, BRAUN-FAHRLANDER C, SCHIERL R, RIEDLER J, EHLERMANN S, MAISCH S, WASER M, NOWAK D. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 21.MARTINEZ FD, HOLT PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354(Suppl 2):SII12–15. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 22.BELLOU A, SCHAUB B, TING L, FINN PW. Toll receptors modulate allergic responses: interaction with dendritic cells, T cells and mast cells. Curr Opin Allergy Clin Immunol. 2003;3:487–494. doi: 10.1097/00130832-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 23.MOORE MM, RIFAS-SHIMAN SL, RICH-EDWARDS JW, KLEINMAN KP, CAMARGO CA, Jr, GOLD DR, WEISS ST, GILLMAN MW. Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics. 2004;113:468–474. doi: 10.1542/peds.113.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FINN PW, BOUDREAU JO, HE H, WANG Y, CHAPMAN MD, VINCENT C, BURGE HA, WEISS ST, PERKINS DL, GOLD DR. Children at risk for asthma: home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105:933–942. doi: 10.1067/mai.2000.106546. [DOI] [PubMed] [Google Scholar]
- 25.FLEMING CM, HE H, CIOTA A, PERKINS D, FINN PW. Administration of pentoxifylline during allergen sensitization dissociates pulmonary allergic inflammation from airway hyperresponsiveness. J Immunol. 2001;167:1703–1711. doi: 10.4049/jimmunol.167.3.1703. [DOI] [PubMed] [Google Scholar]
- 26.ARESTIDES RS, HE H, WESTLAKE RM, CHEN AI, SHARPE AH, PERKINS DL, FINN PW. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32:2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.MARK DA, DONOVAN CE, DE SANCTIS GT, HE HZ, CERNADAS M, KOBZIK L, PERKINS DL, SHARPE A, FINN PW. B7-1 (CD80) and B7-2 (CD86) have complementary roles in mediating allergic pulmonary inflammation and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2000;22:265–271. doi: 10.1165/ajrcmb.22.3.3747. [DOI] [PubMed] [Google Scholar]
- 28.HUANG LY, ALIBERTI J, LEIFER CA, SEGAL DM, SHER A, GOLENBOCK DT, GOLDING B. Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is toll-like receptor 2 dependent. J Immunol. 2003;171:1441–1446. doi: 10.4049/jimmunol.171.3.1441. [DOI] [PubMed] [Google Scholar]
- 29.VARADARADJALOU S, FEGER F, THIEBLEMONT N, HAMOUDA NB, PLEAU JM, DY M, AROCK M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 30.SUPAJATURA V, USHIO H, NAKAO A, AKIRA S, OKUMURA K, RA C, OGAWA H. Differential responses of mast cell toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VAN DER KLEIJ D, LATZ E, BROUWERS JFHM, KRUIZE YCM, SCHMITZ M, KURT-JONES EA, ESPEVIK T, DE JONG EC, KAPSENBERG ML, GOLENBOCK DT, TIELENS AGM, YAZDANBAKHSH M. A novel host-parasite lipid cross-talk. Schistosomal lysophosphati-dylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 32.AGRAWAL S, AGRAWAL A, DOUGHTY B, GERWITZ A, BLENIS J, VAN DYKE T, PULENDRAN B. Cutting edge: different toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 33.MEDZHITOV R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 34.HORNG T, BARTON GM, FLAVELL RA, MEDZHITOV R. The adaptor molecule TIRAP provides signalling specificity for toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 35.HORNG T, BARTON GM, MEDZHITOV R. TIRAP: an adapter molecule in the toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 36.YAMAMOTO M, SATO S, MORI K, HOSHINO K, TAKEUCHI O, TAKEDA K, AKIRA S. Cutting edge: a novel toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 37.YAMAMOTO M, SATO S, HEMMI H, SANJO H, UEMATSU S, KAISHO T, HOSHINO K, TAKEUCHI O, KOBAYASHI M, FUJITA T, TAKEDA K, AKIRA S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 38.POLTORAK A, HE X, SMIRNOVA I, LIU MY, VAN HUFFEL C, DU X, BIRDWELL D, ALEJOS E, SILVA M, GALANOS C, FREUDENBERG M, RICCIARDI-CASTAGNOLI P, LAYTON B, BEUTLER B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 39.PRESCOTT SL, MACAUBAS C, HOLT BJ, SMALLACOMBE TB, LOH R, SLY PD, HOLT PG. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–4737. [PubMed] [Google Scholar]
- 40.PRESCOTT SL. The significance of immune responses to allergens in early life. Clin Exp Allergy. 2001;31:1167–1169. doi: 10.1046/j.1365-2222.2001.01172.x. [DOI] [PubMed] [Google Scholar]
- 41.UPHAM JW, LEE PT, HOLT BJ, HEATON T, PRESCOTT SL, SHARP MJ, SLY PD, HOLT PG. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LA PINE TR, JOYNER JL, AUGUSTINE NH, KWAK SD, HILL HR. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B streptococci. Pediatr Res. 2003;54:276–281. doi: 10.1203/01.PDR.0000072515.10652.87. [DOI] [PubMed] [Google Scholar]
- 43.PRESCOTT SL, JONES CA. Cord blood memory responses: are we being naive? Clin Exp Allergy. 2001;31:1653–1656. doi: 10.1046/j.1365-2222.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 44.DEVEREUX G, SEATON A, BARKER RN. In utero priming of allergen-specific helper T cells. Clin Exp Allergy. 2001;31:1686–1695. doi: 10.1046/j.1365-2222.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 45.HOLT PG, JONES CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 46.WARNER JO, WARNER JA, MILES EA, JONES AC. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:1516. doi: 10.1016/s0140-6736(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 47.WARNER JA, MILES EA, JONES AC, QUINT DJ, COLWELL BM, WARNER JO. Is deficiency of interferon gamma production by allergen-triggered cord blood cells a predictor of atopic eczema? Clin Exp Allergy. 1994;24:423–430. doi: 10.1111/j.1365-2222.1994.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 48.YAZDANBAKHSH M, KREMSNER PG, VAN REE R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 49.MATRICARDI PM, BONINI S. High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the hygiene hypothesis? Clin Exp Allergy. 2000;30:1506–1510. doi: 10.1046/j.1365-2222.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 50.MOREIN B, ABUSUGRA I, BLOMQVIST G. Immunity in neonates. Vet Immunol Immunopathol. 2002;87:207–213. doi: 10.1016/s0165-2427(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 51.HAESAERT B, ORNOY A. Transplacental effects of endotoxemia on fetal mouse brain, bone, and placental tissue. Pediatr Pathol. 1986;5:167–181. doi: 10.3109/15513818609041199. [DOI] [PubMed] [Google Scholar]
- 52.HOLMLUND U, CEBERS G, DAHLFORS AR, SANDSTEDT B, BREMME K, EKSTROM ES, SCHEYNIUS A. Expression and regulation of the pattern recognition receptors toll-like receptor-2 and toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.IKEDA T, FUNABA M. Altered function of murine mast cells in response to lipopolysaccharide and peptidoglycan. Immunol Lett. 2003;88:21–26. doi: 10.1016/s0165-2478(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 54.FROST RA, NYSTROM GJ, LANG CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 55.BOLTE G, BISCHOF W, BORTE M, LEHMANN I, WICHMANN HE, HEINRICH J. Early endotoxin exposure and atopy development in infants: results of a birth cohort study. Clin Exp Allergy. 2003;33:770–776. doi: 10.1046/j.1365-2222.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 56.BOTTCHER MF, BJORKSTEN B, GUSTAFSON S, VOOR T, JENMALM MC. Endotoxin levels in Estonian and Swedish house dust and atopy in infancy. Clin Exp Allergy. 2003;33:295–300. doi: 10.1046/j.1365-2222.2003.01562.x. [DOI] [PubMed] [Google Scholar]
- 57.LITONJUA AA, MILTON DK, CELEDON JC, RYAN L, WEISS ST, GOLD DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110:736–742. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 58.PARK JH, GOLD DR, SPIEGELMAN DL, BURGE HA, MILTON DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 59.SCHWARTZ DA. The role of TLR4 in endotoxin responsiveness in humans. J Endotoxin Res. 2001;7:389–393. [PubMed] [Google Scholar]


