Abstract
The adult hippocampal dentate gyrus (DG) is a site of continuing neurogenesis. This process is influenced by a variety of physiological and experiential stimuli including total sleep deprivation (TSD). In humans, sleep fragmentation (SF) is a more common sleep condition than TSD. SF is associated with several prevalent diseases. We assessed a hypothesis that SF would suppress adult neurogenesis in the DG of the adult rat. An intermittent treadmill system was used; the treadmill was on for 3 s and off for 30 s (SF). For sleep fragmentation control (SFC), the treadmill was on for 15 min and off for 150 min. SF was conducted for 3 durations: 1, 4 and 7 days. To label proliferating cells, the thymidine analog, BrdU, was injected two hours prior to the end of each experiment. Expression of the intrinsic proliferative marker, Ki67, was also studied.
SF rats exhibited an increased number of NREM sleep bouts with no change in the percent of time spent during this stage. The numbers of both BrdU-positive cells and Ki67-positive cells were reduced by ~ 70 % (P < 0.05) in the SF groups after 4 and 7 days of experimental conditions whereas no differences were observed after 1 day. In a second experiment, we found that the percentage of new cells expressing a neuronal phenotype three weeks after BrdU administration was lower in the SF in comparison with the SFC group for all 3 durations of SF. We also examined the effects of SF on proliferation in adrenalectomized (ADX) animals, with basal corticosterone replacement. ADX SF animals exhibited a 55% reduction in the number of BrdU-positive cells when compared with ADX SFC. Thus, elevated glucocorticoids do not account for most of the reduction in cell proliferation induced by the SF procedure, although a small contribution of stress is not excluded. The results show that sustained SF induced marked reduction in hippocampal neurogenesis.
Keywords: Sleep fragmentation, hippocampus, dentate gyrus, neurogenesis, BrdU
The subgranular cell layer (SGZ) of the hippocampal dentate gyrus (DG) contains progenitor cells that have the potential to generate new neurons throughout life, a phenomenon referred to as adult neurogenesis (Kuhn et al., 1996). Adult neurogenesis has been documented in birds, rodents, and primates, including humans (Goldman and Nottebohm, 1983; Altman and Das, 1965; Eriksson et al., 1998). Adult neurogenesis comprises sequential processes wherein proliferating cells survive, differentiate into neurons, migrate, and generate axons and dendrites. These processes are subject to either augmentation or suppression by a variety of physiological and experiential stimuli (Kempermann et al., 1998; Lledo et al., 2006). Sustained total sleep deprivation is a potent suppressor of neurogenesis (Guzman-Marin et al., 2003, 2005, Tung et al., 2005).
In humans, sustained total sleep deprivation is rare, but several prevalent diseases are associated with sleep fragmentation. Severe sleep fragmentation is characteristic of obstructive sleep apnea (OSA; Issa and Sullivan 1986) and periodic leg movements of sleep (Sforza et al, 1999), and lesser sleep fragmentation (SF) is characteristic of aging (Bliwise. 1993). SF is characterized by repetitive short interruptions of sleep. These arousals do not result in prolonged wakefulness (Bonnet and Arand 2003). Sleep latency values are typically reduced by 60% or more after one night of either total sleep deprivation (TSD) or SF. Some physiological parameters like cortisol measurements have been shown to exhibit similar profiles following either TSD or SF (Spath-Schwalbe et al., 1991). In human studies, it has been shown that if sleep was fragmented by brief awakenings at one-minute intervals, daytime performance and vigilance task performance was impaired the next day to virtually the same extent as after TSD (reviewed in Bonnet and Arand 2003). Given that short term SF and TSD have similar effects in humans, we assessed a hypothesis that SF, like total sleep deprivation, would suppress adult neurogenesis in a rodent model. To assess the effects of SF on proliferation, we used both the exogenous marker, BrdU, which labels cells in the S-phase of the cells cycle, and is retained for long-term labeling, and Ki-67, a transient endogenous marker expressed in all phases of the cell cycle, except the rest phase. Given that corticosterone release associated with stress is a potent negative regulator of proliferation (Cameron and Gould 1994; Heine et al., 2004), and SF could be stressful (Mirescu et al, 2006), we also examined the effects of SF on proliferation in adrenalectomized animals, with basal corticosterone replacement.
Experimental procedures
Animal preparation
All protocols were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Animal Care and Use Committee at the V.A.G.L.A.H.S. Male Sprague-Dawley rats (300 – 320 g) were used for this experiment. Animals were housed individually in Plexiglas cages (27×29×30cm) in a 12:12 light/dark cycle with access to water and food ad libitum. Under deep anesthesia (Ketamine 80 mg kg−1, i.p + Xylazine 10 mg kg−1, i.p.) and aseptic conditions rats were surgically prepared for chronic polysomnographic recording, as described previously (Guzman-Marin et al., 2003). Briefly, five steel stainless screw electrodes were implanted in the skull (frontal and parietal bones) for electroencephalogram (EEG) recording, and 4 steel stainless wires were inserted into the muscles of the dorsal part of the neck for electromyogram (EMG) recording. All electrodes were connected to pins in a plug assembly, which was fixed to the skull with dental acrylic. Rats were allowed a 7-day recovery period following surgery.
Sleep fragmentation paradigm
To achieve sleep fragmentation (SF) an intermittent treadmill system was used. This assembly consisted of a plastic chamber (28cm×28cm×40cm), which is fixed and suspended 0.6 cm above the vinyl belt of a treadmill, the belt forming the floor of the cage. Food and water were available at all times while the animals were on the treadmill, and the timing of the light/dark cycle was the same as in the home cage. The treadmill speed was set at 10 cm s−1. Rats were divided into two groups of 6 rats each: SF and sleep fragmentation control (SFC). Two rats from each group were studied concurrently. SF was achieved by automated activation of the treadmill for 3 consecutive seconds at 33 sec intervals (i.e., 3 sec on; 30 sec off). Activation of the treadmill caused the animal to step to avoid being carried into the wall of the chamber. For the control procedure the treadmill was activated for 15 min and off for 150 min, allowing the SFC animals unrestricted sleep for 150 min at 165 min intervals. This schedule allowed SFC to achieve sustained periods of rest but equated the total distance moved in SF and SFC animals. The effectiveness of SF versus SFC procedures was verified by continuous recordings of EEG and EMG activity while rats were on the treadmill. Between each use, the treadmill belt and cage were scrubbed with detergent, wiped with a bleach solution and rinsed with water.
Sleep-wake parameter recording procedures
For sleep-wake cycle recordings on the treadmills or in home cages, rats were connected to amplifiers through light cables suspended by an overhead counterbalanced cable and slip ring. For several consecutive days before beginning the SF protocol, rats were acclimated to the recording procedures.
EEG and EMG signals were filtered at 1.0 and 30 Hz and 30–300 Hz, respectively, and digitized with the Gamma sleep recording and analysis software system (Grass Telefactor, West Warwick RI, USA). Sleep-wake states were scored in 10 s epochs on the basis of the predominant state within the epoch. Wake was identified by low-voltage high-frequency EEG activity and sustained elevated neck muscle tone. High-amplitude low frequency EEG with decreased muscle activity defined NREM sleep whereas REM sleep was defined by moderate-amplitude EEG with dominant theta frequency (4–8 Hz), combined with low muscle tone. The percentages of each state were calculated for the total recording period (1, 4 and 7 days).
In addition to determining the total duration of each sleep-wake state over the entire course of the experiment, we measured the number and duration of sleep bouts to access the level of sleep fragmentation. The duration of sleep bouts in each rat was calculated for each 24 h period.
Experimental design
Experiment 1 evaluated the effects of varying durations of SF (1,4 or 7 days) on cell proliferation. Rats were surgically implanted for polysomnographic recordings and divided into SF and SFC groups (n=6 each). Experiments were carried out as described above. At the end of days one, four or seven at ZT 0 (beginning of light phase, 08:00), all animals were injected with BrdU (i.p. 300 mg/kg dissolved in 0.9% NaCl) and were euthanized and perfused 2 h later. SF or SFC procedures were maintained during the last 2 hours.
Experiment 2 examined the phenotype of the newly generated cells. Separate groups of rats (n = 6 each) were prepared and subjected to SF and SFC, as in experiment 1. After 1, 4 and 7 days of experimental manipulations, a single dose of BrdU (300 mg/kg, i.p.) was given, and rats were returned to their home cages. After 3 weeks animals were perfused.
Experiment 3 determined if the reduction in cell proliferation after prolonged SF was mediated by elevation of corticosterone (CORT). Age matched adrenalectomized (ADX) rats were purchased from Charles River Laboratories (Hollister, California USA). The effectiveness of ADX was confirmed by visual inspection when animals were perfused. To maintain plasma sodium balance and basal CORT levels, ADX animals were given CORT in drinking water (25 μg/ml in 0.9% NaCl; Sigma). Following surgery, recovery, and adaptation as in experiment 1, ADX experimental and control groups (n=6 each) were exposed to 4 days of SF or SFC procedures, and were then injected with BrdU and perfused 2 h later. For technical reasons, CORT levels were not obtained.
Perfusion, immunohistochemistry, and cell counting
Subjects from all groups were deeply anesthetized (Nembutal 100 mg kg −1), perfused transcardially with PB 0.1 M followed by ice cold paraformaldehyde (4%); brains were removed and stored in 10% and 30% sucrose at 4°C until they sank. Brains were cut in 40 μm coronal sections. Sections encompassing the hippocampus were preserved in a cryoprotectant solution containing sucrose, polyvinyl-pyrrolidone (PVP-40, Sigma) and ethylene glycol dissolved in PB pH 7.2, which provides long-term protection of the tissue. Sections were processed for BrdU immunohistochemistry or triple immnunofluorescence labeling to identify cell phenotype. As primary antibodies we used: mouse anti BrdU (1:400 Novocastra), rat anti-BrdU (1:100; Accurate), mouse anti-NeuN (1:300 Chemicon) and rabbit anti S100-β (1:2500, Swant, Bellinoza, Switzerland).
For single labeling, to visualize the expression of the BrdU alone we used the peroxidase method (ABC system, Vectastain, Vector Laboratories). Immunohistochemistry was performed simultaneously on sections from CT and SD rats to maximize the reliability of comparisons across groups. Staining was performed on free-floating sections. A one-in-six series of sections was pretreated for BrdU immunostaining by DNA denaturation (2 M HCl at 37 °C for 30 min) followed by 10 min in borate buffer (pH 8.5). Tissue was rinsed in TBS 0.1M. Sections were then incubated with a mouse anti-BrdU primary antibody for 48 h. Tissue from both groups was treated with aliquots from the same batch of antibodies. Sections were subsequently incubated with a biotinylated horse anti-mouse IgG (1:200, Vector Laboratories), then reacted with avidin–biotin complex (1:100, Vector Elite) and developed with diaminobenzidine tetrahydrochloride (DAB, Sigma). Absence of the primary antibody resulted in an absence of specific nuclear staining.
A separate set of sections (one of six) from the same brains used for BrdU immunostaining was processed for Ki-67 immunostaining in slide-mounted sections (Heine et al., 2004) using rabbit anti-Ki-67 (1:1000, Novocastra) as the primary antibody and the peroxidase method to visualize the Ki-67 expression.
BrdU- and Ki-67-positive cells were counted using a 40x objective (Nikon E600) throughout the rostrocaudal extent of the DG granule cell layer. The optical fractionator method was used for counting as previously described (Guzman-Marin et al., 2005). Briefly, in every section, the contour of GCL/SGZ area was first delineated for counting using tracing function of the Stereo Investigator. Following this, the optical fractionator component was activated by entering parameters such as the grid size, the thickness of guard zone (5 μm) and the optical dissector height (Guzman-Marin et al. 2003). A computer-driven motorized stage then determined counting frame locations randomly. The StereoInvestigator software calculated the total number of BrdU and Ki-67 positive cell per DG by utilizing the optical fractionator formula. A total of 18–20 sections per brain were analyzed. This procedure ensured a systematic random sample of sections, in which all parts of the DG region analyzed had the same opportunity of being represented. The precision of estimates of the number of cells was expressed using the coefficient of error (CE). The stereological sampling scheme was considered adequate when CE was less than 0.10 (West and Gundersen, 1990).
The phenotype of the newly generated BrdU-positive cells was estimated using triple immunolabeling with antibodies to BrdU, NeuN, and the glial calcium-binding protein, S100-β. A one-in-12 series of sections from animals of each group were processed for triple labeling with BrdU, NeuN and S100β. After pretreatment (see above) and blocking with goat serum and triton-X 10% in TBS, sections were incubated in a mixture with antibodies against BrdU (monoclonal from rat), NeuN (monoclonal from mouse) and S100β (monoclonal from rabbit) in TBS for 3 days at 4° C. After rinsing with TBS and blocking for 1 h, sections were incubated in an antibody mixture of Alexa 488 Goat anti-rat and Alexa 567 Goat anti-mouse and Alexa 633 Goat anti-rabbit all at 1:300 (Molecular Probes) in TBS for 2 h. Sections were mounted and cover-slipped with Vectastain (Vector) mounting medium.
The phenotype of newly generated cells within the granule cell layer was assessed by double labeling of BrdU and NeuN or BrdU and S100β with a fluorescence microscope (Nikon E-400) and a Leica TCS SP MP fixed-stage upright confocal microscope using argon, krypton and helium-neon lasers. Analysis was performed in sequential scanning mode, where only one laser and its respective detection line (488, 567 or 633 nm) are active at a time to exclude cross bleeding between the two or three different channels. Co-localization was confirmed by z-series analysis through the cell nucleus and three-dimensional reconstruction that allows viewing of cells in the x-z and y-z direction (z-step, 1 μm). Co-localization was confirmed in 50 cells per animal by z-series analysis through the cell nucleus and three-dimensional reconstruction. An individual blinded to the experimental condition did all counting. Photomicrographs of immunofluorescent staining were generated by using a Leica TCS SP MP fixed-stage upright confocal microscope and stored as RGB Tiff files. Picture montage and adjustment for brightness and contrast were performed by using Photoshop 7.0 (Adobe Systems, Inc., San Jose, CA). For comparability, all images on were adjusted with identical contrast and brightness settings.
Statistical Analysis
To assess the impact of the sleep fragmentation paradigm on different sleep wake cycle parameters between and within groups, a repeated-measures ANOVA was carried out, with days of treatment as the repeated-measures variable followed by Tukey’s post hoc test. Cell count differences between groups were assessed with Student’s t-test. A P value of < 0.05 was adopted for significance.
Results
Effects of 1, 4 or 7 days SF on sleep parameters
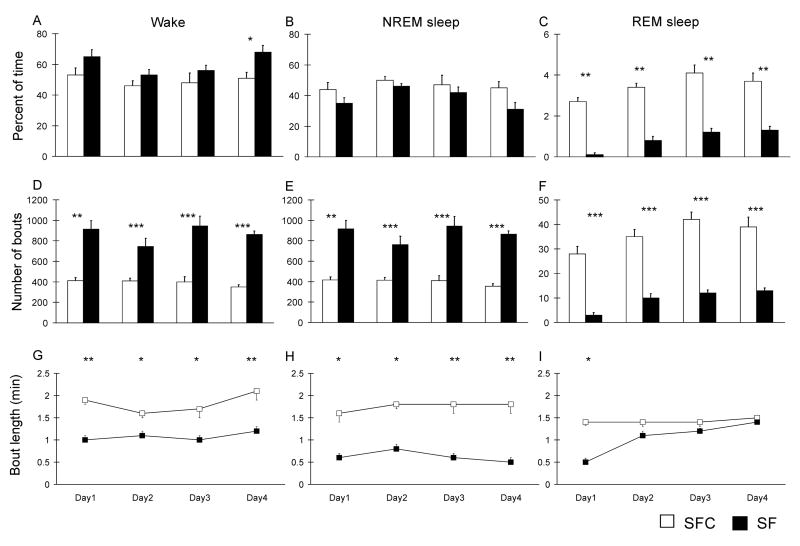
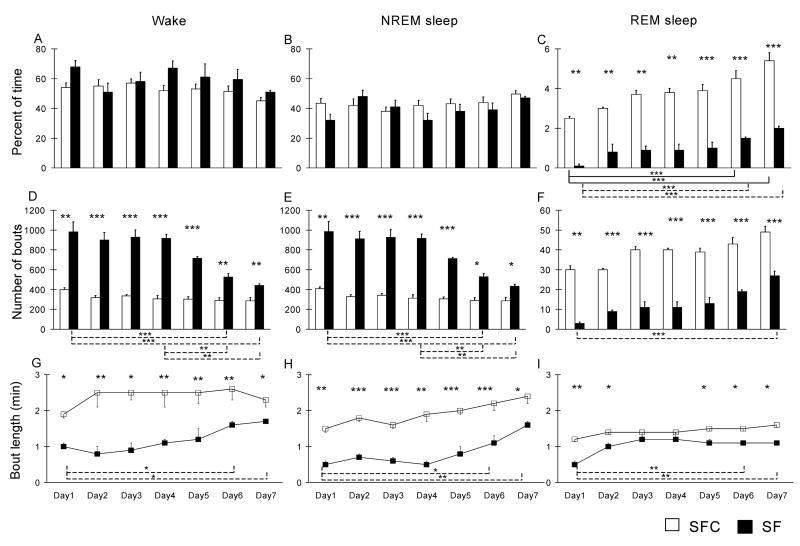
SF resulted in small increases in percent time spent in waking, ranging from 13% in the 7-day treatment group to 25–30% in the 1 and 4-day treatments (Fig. 1A, Fig. 2A), but this increase was significant only on day 4 in the 4 day SF treatment, and on days 4 in the 7 day treatment. The percentage of NREM sleep was not significantly changed (Fig. 1B, Fig. 2B). REM sleep was almost completely suppressed by one day of SF and was markedly reduced in the 4-day (groups: F(1,10) = 115.5, P < 0.001) and 7-day (groups: F(1,10) = 175.6, P < 0.001) treatment groups (Fig. 1C, Fig. 2C). However, in the 7-day treatment group there was a progressive increase across days of treatment in the percent of time spent during REM sleep (days: F(6, 60) = 26.9, P < 0.001); the percentage of REM sleep was significantly greater on day 7 in the 7-day treatment group than on day 4 in that group. REM bout length also increased during the course of SF (Fig. 1I, 2I) in the 7-day group (groups: F(1,10) =11.12, P < 0.01; days: F(6, 60) = 6.9, P < 0.001).
Figure 1. Effects of 4 days of sleep fragmentation on the sleep-wake cycle.
The graphs show the percentage of time spent in the different stages of the sleep-wake cycle (A, B and C) in sleep fragmentated animals (SF) and their controls (SFC) during each day of recording. The number of bouts (D, E and F) and the mean bout duration (G, H and I) were quantified for each stage of the sleep wake cycle. Data are means ± S.E.M. for groups of 6 rats each. *** P < 0.001, ** P < 0.01 and * P < 0.05, Student’s t-test.
Figure 2. Effects of 7 days of sleep fragmentation on the sleep-wake cycle.
The graphs show the percentage of time spent in the different stages of the sleep-wake cycle (A, B and C) in sleep fragmentated animals (SF) and their controls (SFC) during each day of recording. The number of bouts (D, E and F) and the mean bout duration (G, H and I) were quantified for each stage of the sleep wake cycle. Note that in this treatment group there was a progressive increase in the percent of time spent during REM across days of treatment. Data are means ± S.E.M. for groups of 6 rats each. *** P < 0.001, ** P < 0.01 and * P < 0.05, Student’s t-test.
In addition to determining the total duration of each sleep-wake state over the course of the experimental procedures, we quantified the number and length of wake, NREM and REM sleep bouts in animals subjected to 4 and 7 days of experimental and control procedures. The duration of sleep bouts in each rat was calculated for each 24 h period and the total period of recording. SFC rats had longer periods of uninterrupted sleep (Fig. 3) as compared with SF animals. SF rats had twice as many NREM sleep bouts as SFC rats (Fig. 1B, Fig. 2B) both in the 4-day groups (groups: F(1, 10) =150.7, P < 0.001) and 7-day groups (groups: F(1,10) = 151.5, P < 0.001); this fragmentation of sleep in SF rats was manifested by a significant reduction in the duration of episodes of NREM sleep, (groups: F(1, 10) = 30.6 P < 0.001 in the 4 days group; groups: F(1, 10) = 924.4, P < 0.001 in the 7 days group) by about 50% (Fig. 1H, Fig. 2 H). SF rats also had a significant increase in the number of wakefulness episodes (groups: F(1, 10) = 157.4 p < 0.001 in the 4 days group; groups: (F(1, 10) = 150 p < 0.001 at 7 days) and a significant reduction in the duration of these episodes.
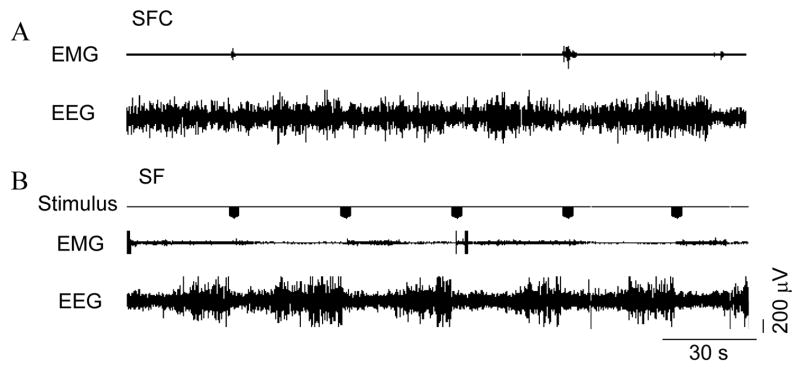
Figure 3. Simultaneous polysomnographic recording from a SF rat and its control (SFC).
In SF animals treadmill is activated throughout the entire recording session in a 3 seconds on/30 seconds off repetitive cycle whereas the system is activated for 15 min on/150 minutes off in the SFC animals. EEG: electroencephalogram; EMG: electromyogram.
Effects of 1, 4 and 7 days SF on cell proliferation
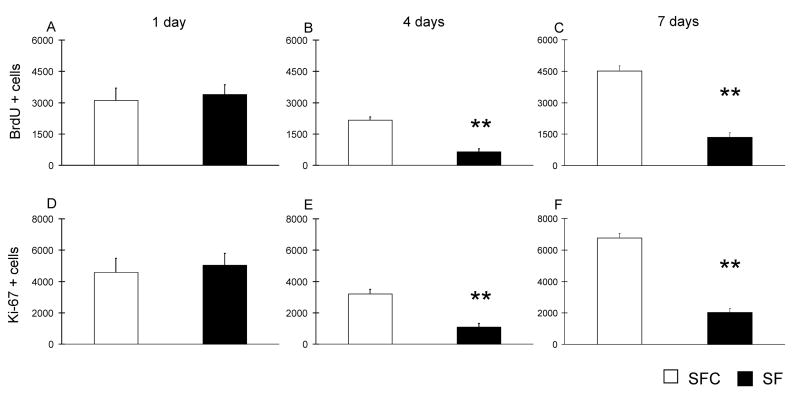
We first investigated whether the proliferative stage of neurogenesis in the hippocampal DG is affected by SF treatment. In the 1-day group no differences were observed between the SF and SFC groups. Following both 4 and 7 day SF treatments (Fig. 4, B–C), we observed a ~70% reduction in the number of BrdU-positive cells (p < 0.01, t = 3.4; and P < 0.001, t = −4.7, df 11, respectively). In addition, different sections from the same brains used for BrdU counts were evaluated for expression of Ki-67. The cell counts profile was similar to that of BrdU cells, in that no difference was observed following 1-day of SF, but 68 and 69% reductions were found in the number of Ki-67 cells (Fig. 4, E–F) in SF rats when compared to SFC after 4 and 7 days (P < 0.05, t = −3.2; and P < 0.01, t = −4.1, df respectively).
Figure 4. Effects of sleep fragmentation on proliferation of cells in the DG.
Cell proliferation was assessed by counting the total number of BrdU- or Ki-67-positive cells per DG at 1 (A and D), 4 (B and E) and 7 days (C and F). Both the number of BrdU-and Ki-67-positive cells in the DG of the dorsal hippocampus were reduced in the SF group in comparison with the SFC after 4 and 7 days of treadmill treatments. Data are means ± S.E.M. for groups of 6 rats each. ** P < 0.01, Student’s t-test.
Effects of 1, 4 and 7 days of SF on phenotype of newly generated cells
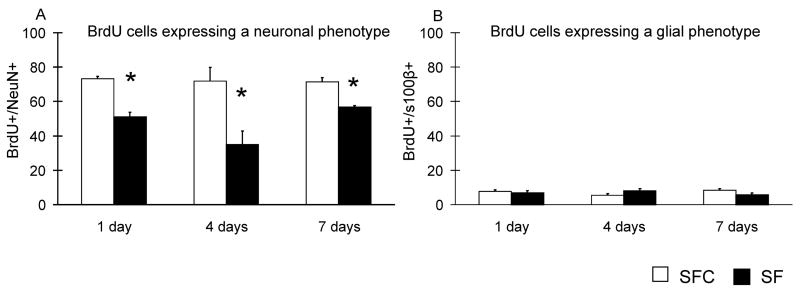
The phenotype of the surviving BrdU-positive cells was evaluated 3 weeks after the administration of BrdU by immunofluorescent triple labeling for BrdU, the neuronal marker NeuN (Fig. 5A) and the glial marker S100β (Fig. 5B). The mean percentages of the BrdU/NeuN-positive cells differed significantly between the groups (Fig. 6). The percentages of BrdU-positive cells co-labeled with NeuN in animals subjected to 1 and 7 days of SF were 30% and 22% lower than in SFC animals (P < 0.001 and P < 0.05, respectively). In the 4 days group, we observed a 52% reduction of double-labeled cells in the SF when compared with SFC group. The localization of BrdU/NeuN-positive cells was similar in all groups (Fig. 7). There were no differences between the groups with respect to the percentage of BrdU-positive cells that expressed the glial phenotype (Fig. 6 B). The percentages of cells that did not co-localize BrdU with either NeuN or S100β did not differ significantly between SF and SFC animals.
Figure 5. Effects of sleep fragmentation on phenotype expression of the surviving cells.
Phenotypes of the surviving cells were determined by immunofluorescent triple labeling for BrdU, NeuN (neurons) and S100β (glia) three weeks after the administration of BrdU. The percentages of BrdU-positive cells co-labeled for either NeuN (A) or S100β (B) are presented. * P < 0.05, Student’s t-test.
Figure 6. Effects of sleep fragmentation on cell proliferation in ADX rats.
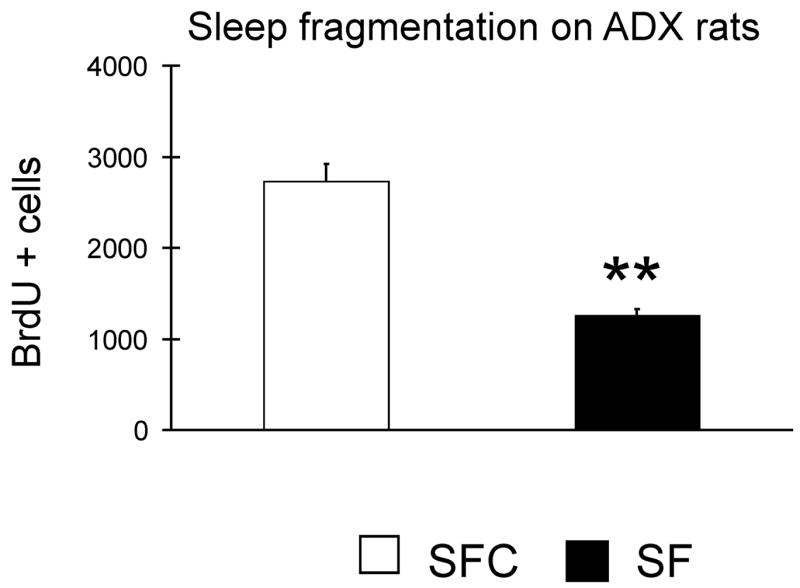
The effects of 4 days of experimental manipulations in ADX animals receiving basal level corticosterone in drinking water was tested. ADX SF animals exhibited a 55% reduction in the number of BrdU positive cells when compared with ADX SFC ** P < 0.01, Student’s t-test.
Figure 7.
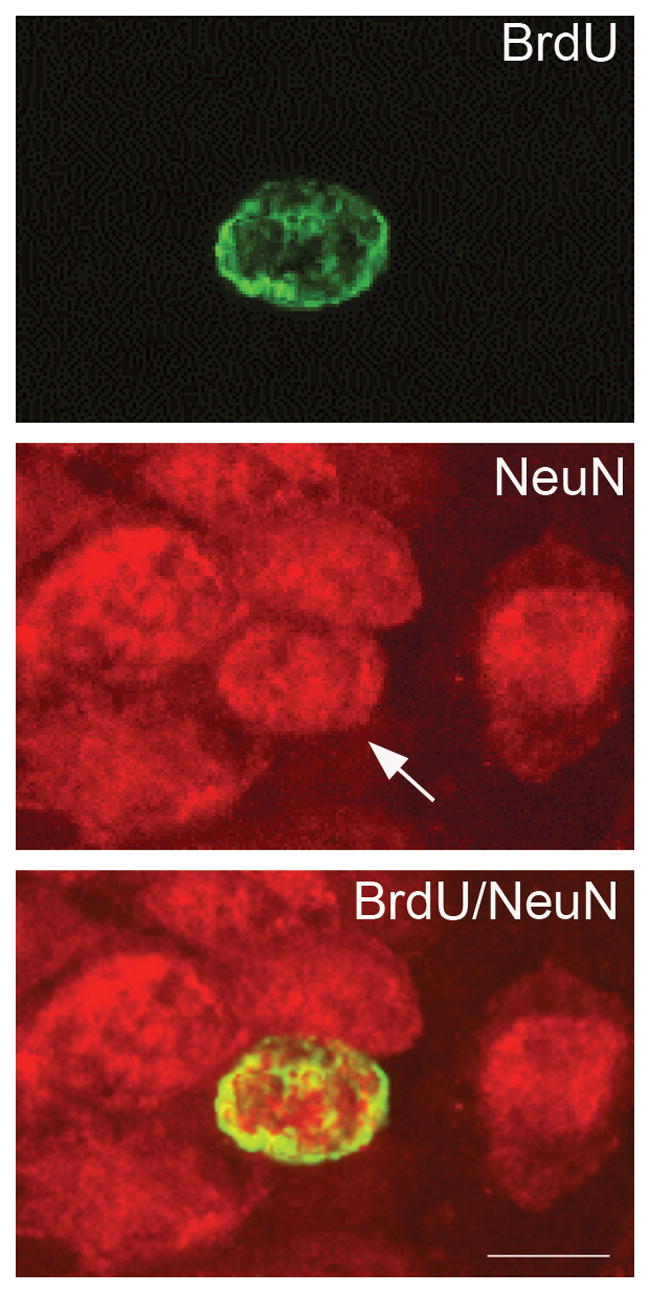
A newly generated cell in the dentate gyrus expressing neuronal phenotypic marker 3 weeks after BrdU injection, as detected by immunofluorescence double-label and confocal microscopy. Neurons in the dentate gyrus express NeuN (red). The same cell in the GCL that is labeled with BrdU (green) also expresses NeuN (arrow).
Effects of 4 days of SF on cell proliferation in ADX rats
To determine if increased CORT was responsible for the suppression of cell proliferation by SF, rats were adrenalectomized bilaterally (ADX) and maintained on low-dose CORT replacement via CORT in 0.9% saline. After 4 days of experimental interventions (Fig. 7) the number of BrdU- positive cells was significantly reduced in the SF rats ~55% when compared with SFC (P < 0.001, t = −5.6, df 9). This reduction was not significantly different from that found at 4 days in animals with intact adrenal glands (P >0.05, t = 1.89; df 10).
In a separate group of ADX animals we quantified daily water consumption under SF and SFC conditions. During four days of treatment, daily water consumption was comparable in these two conditions (65.5 ± 13 ml in SF vs 66.3 ± 6.2 ml in SFC).
Discussion
The main finding of this study was that sustained sleep fragmentation dramatically reduced cell proliferation in the DG of the hippocampus. We observed this reduction after 4 and 7 days of SF, but no difference was observed after 24 h SF. The reductions observed in the number of BrdU-positive cells were mirrored by reductions in Ki-67 positive cells, ruling out the possibility that changes in the number of BrdU-positive cells could be related to differences in distribution or availability of BrdU during experimental manipulations. The degree of suppression of proliferation after 4 days SF was comparable to that following 4 days TSD that we reported previously (Guzman-Marin et al., 2003). Previous short-term deprivation studies have yielded variable results. Ten–twelve hours of sleep deprivation by gentle handling did not change proliferation in mice (van der Borgth et al., 2006) but increased proliferation in rats (Grassi Zucconi et al., 2006), and, in rats, 24 hrs sleep deprivation by forced activity decreased proliferation in the hilus (Roman et al., 2005), but 24 hr deprivation using the small pedestal over water method did not change proliferation in the DG (Mirescu et al., 2006). Thus, including ours, 3 of 5 studies found no effect of 12–24 h deprivation, the other two reaching contradictory conclusions.
We also examined the phenotype of the surviving BrdU-positive cells 3 weeks after the BrdU injection. In the control group, the majority (~70%) of the newly formed cells in the dentate gyrus expressed the mature neuronal marker, NeuN, a finding that is consistent with other studies (van Praag et al., 1999; Malberg et al., 2000). The percentages of BrdU-positive cells that co-labeled with NeuN were reduced by 52 and 22% in 4 and 7-day SF treatment groups, respectively. These effects on cell fate are consistent with those reported by Hairston et al., (2005) using a sleep restriction paradigm. In the 24 h groups we observed a 30% reduction in percentage of BrdU/NeuN cells when compared with SFC. This finding is consistent with a previous preliminary report indicating no changes in proliferation after 24 h of TSD but a reduction in the number of cells expressing a neuronal marker (Leasure et al., 2003). SF did not affect the percentage of cells exhibiting the glial marker, S100β. Therefore, the SF treatment must alter proliferating cells, impairing their capacity for later differentiation into neurons.
In our study, experimental and control animals experienced the identical total treadmill movement. The treadmill speed was 10 cm/s, a walking rate. Total treadmill movement per day was 785 m, well below that the 4+ km rats will run given access to a running wheel. It has been shown that either voluntary or forced exercise increased neurogenesis (van Pragg et al, 2005, Ra et al., 2002), whereas SF has the opposite effect. A limitation of the present study is that is does not mimic human disease in that SF was applied continuously rather than being sleep-dependent.
Since many of the negative regulators of neurogenesis have in common the influence of stress-related elevation of glucocorticoids (Cameron and Gould 1994; Heine et al., 2004), and the suppression of proliferation by 3 days REM and partial NREM deprivation using the small-pedestal-over-water method has been attributed to elevated glucocorticoids (Mirescu et al., 2006), we examined the role of glucocorticoids in effect of SF on proliferation of DG cells. We examined the effects of 4 day SF and SFC procedures in ADX animals receiving corticosterone replacement in drinking water, considering that the 4-day SF treatment produced the greatest reduction in the number of cells expressing a neuronal phenotype. ADX SF animals exhibited a 55% reduction in the number of BrdU-positive cells when compared with ADX SFC. Intact SF animals exhibited a 70% reduction in proliferation. The difference in the reduction of proliferation in ADX and intact groups was not significant. Thus, SF accounts for most of the reduction in cell proliferation induced by the 4 days exposure to SF procedure, although a small contribution of stress is not excluded.
Methodological differences might explain the discrepancy between the findings of the present study and those of Mirescu et al. with respect to the role of corticosterone. Our SF treatment lasted 4 days, compared to 3 days in Mirescu et al. The SF procedure permits normal amounts of NREM sleep, whereas, the pedestal method used by Mirescu et al initially suppresses NREM as well as REM (Machado et al, 2004). The single pedestal method, used by Mirescu et al, requires continuous postural muscle support and combines physical restraint with sleep disruption, and results in persistent elevations of CORT in SD animals compared to controls (Suchecki et al, 1998). Application of SD methods that do not impose restraint, or require continuous postural support, including intermittent treadmill sleep deprivation (Guzman-Marin et al., 2003) and disk-over-water deprivation methods, (Everson and Crowley, 2004) do not result in differential elevation of CORT, at least after 4 days of deprivation. In our study ADX animals received CORT replacement in their drinking water, because this method has been shown to mimic the normal circadian pattern of CORT secretion (Jacobson et al., 1988). Suchecki et al., (2003) showed that 4 days sleep disruption using the pedestal method did not increase consumption of unsweetened water, and there was little circadian variation in water consumption. In the present study water intake was comparable in SF and SFC conditions; daily water consumption was consistent with that reported in ADX animals in basal conditions (Edmonds, 1960). In addition ADX animals were randomly assigned to SF or SFC groups. It is unlikely that variations in the effectiveness of the ADX procedure would differentially affect these groups.
An additional difference between studies was the time of BrdU administration, at ZT0 in our study, ZT7 in Mirescu et al. However, circadian timing is unlikely to account for differences between studies as we also found suppression of proliferation by SF based on Ki-67 expression counts. Ki-67 labels proliferating cells throughout most of the cell cycle, and would not selectively sample one part of the circadian cycle.
The degree of suppression of proliferation was less after 7 days compared to 4 days SF. The deficit in the percentage of new cells later expressing a mature neuronal phenotype was reduced even more in the 7-day compared to the 4-day treatment. There was also a lesser suppression of REM sleep in the 7-day SF animals compared to 4 days, presumably reflecting increased homeostatic drive for REM. We have recently shown that selective REM sleep deprivation resulted in potent suppression of proliferation (Guzman-Marin et al., 2005). The partial recovery of proliferation as well as the partial recovery of capacity for cell differentiation may have resulted from increased REM. Alternatively, other compensatory processes may be involved, and these may be different for the proliferative and maturational processes. We note that the recovery of proliferation at 7 days is consistent with a previous report (Gozal et al., 2003) using the intermittent hypoxia paradigm, which is accompanied by sleep fragmentation.
A fragmented pattern of sleep is manifested in SF rats by a reduction in the duration of NREM sleep episodes with little change in the percent of time spent during NREM sleep. SF rats showed large increases in the number of short NREM bouts, which is consistent with the increased number of sleep bouts observed in humans subjected to SF (Bonnet and Arand, 2003). In SF animals we also observed a significant reduction in the REM sleep at all treatment durations studied. In the present study the suppression of proliferation and potential for cell maturation in the DG could have resulted from either SF, that is, reduced sleep and waking bout duration or from reduction of REM sleep, or both.
SF is defined by increased arousals during sleep. Arousals do not result in sustained awakenings. However, experimental SF results in increased daytime sleepiness (Bonnet 1985; Levine et al., 1987; Martin et al., 1999; Stepanski et al., 1987) cognitive performance deficits (Bonnet 1986; Martin et al., 1996) and mood alterations (Bonnet 1987, Bonnet el al. 1991; Kingshott et al., 2000). The same deficits have been found in patients with OSA associated with severe SF (reviewed by Durmer and Dinges, 2005). SF also results in spatial learning and attention set shifting deficits in rats (Tartar et al, 2006; McCoy et al, 2007). These studies show that SF has deleterious effects on waking behavior comparable to those produced by total sleep deprivation, and suggest that the restorative effects of sleep depend on occurrence of sustained sleep episodes (Bonnet 1985, 1986, 1987). One analysis showed that SF attenuated the lowering of metabolic rate associated with more sustained sleep episodes (Bonnet et al, 1991). This analysis is congruent with concepts that sleep functions to restore cellular energy supply (Bennington and Heller 1995; Basheer et al., 2004).
Sleep fragmentation is a characteristic of sleep in normal aging (Bliwise, 2004) and pathological conditions such as OSA (Issa and Sullivan 1986), which are also associated with a reduction in hippocampal volume (Raz, 1998; Macey et al., 2002; Morrell et al., 2003). While stress or other factors may contribute to hippocampal changes in these diverse conditions, suppression of adult neurogenesis due to SF may be one of the mechanisms underlying hippocampal volume reduction found in association with these conditions.
Acknowledgments
We gratefully acknowledge Feng Xu and Keng-Tee Chew for their excellent assistance. This research was made possible by a grant from the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine to RGM. Supported by the US Department of Veterans Affairs Medical Research service and US National Institutes of Health grants NIMH 075076, HL 60296.
Abbreviations
- ADX
adrenalectomized rats
- BrdU
5-bromo-2-deoxyuridine
- CORT
corticosterone
- SF
sleep fragmentation
- SFC
sleep fragmentation control
- DG
dentate gyrus
- EEG
electroencephalogram
- EMG
electromyogram
- GCL
granule cell layer
- NREM
non-rapid eye movement
- PB
phosphate buffer
- REM
rapid eye movement
- REMD
rapid eye movement sleep deprivation
- SGZ
subgranular cell layer
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Auto radiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias (2004) Clin Cornerstone. 2004;6:S16–28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia (1993) Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71:1112–1118. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Rosa RR. Sleep and performance in young adults and older normals and insomniacs during acute sleep loss and recovery. Biol Psychol. 1987;25:153–72. doi: 10.1016/0301-0511(87)90035-4. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–373. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–271. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–19. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Sem Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Edmonds CJ. Fluid intake and exchangeable body sodium of normal and adrenalectomized rats given various concentrations of saline to drink. Q J Exp Physiol Cogn Med Sci. 1960;45:163–70. doi: 10.1113/expphysiol.1960.sp001454. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab. 2004;286:E1060–1070. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, Sachleben LR, Guo SZ. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. Eur J Neurosci. 2003;18:2335–2342. doi: 10.1046/j.1460-9568.2003.02947.x. [DOI] [PubMed] [Google Scholar]
- Grassi Zucconi GG, Cipriani S, Balgkoiranidou I, Scattoni R. ‘One night’ sleep deprivation stimulates hippocampal neurogenesis. Brain Res Bull 2006. 2006;69:375–381. doi: 10.1016/j.brainresbull.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Bashir T, Suntsova N, Nienhuis R, Szymusiak R, McGinty D. 2005 Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2005. 2005. Selective REM sleep deprivation reduces cell proliferation in the dentate gyrus of the hippocampus in the adult rat. Program No. 309.14. [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–6. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Stewart DR, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–71. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Heine V, Maslam S, Joels M, Lucassen P. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Issa FG, Sullivan CE. The immediate effects of nasal continuous positive airway pressure treatment on sleep pattern in patients with obstructive sleep apnea syndrome. Electroencephalogr Clin Neurophysiol. 1986;63:10–17. doi: 10.1016/0013-4694(86)90056-8. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Shinako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–12. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingshott RN, Cosway RJ, Deary IJ, Douglas NJ. The effect of sleep fragmentation on cognitive processing using computerized topographic brain mapping. J Sleep Res. 2000;9:353–357. doi: 10.1046/j.1365-2869.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- Kuhn GH, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Hardy MN, Chiappinelli BB, Bazan NG, Gage FH. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; Sleep deprivation alters phenotype of hippocampal progenitor cells: influence of exercise. Program No. 561.4. [Google Scholar]
- Levine B, Roehrs T, Stepanski E, Zorick F, Roth T. Fragmenting sleep diminishes its recuperative value. Sleep. 1987;10:590–599. [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb M. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SE, Brander PE, Deary IJ, Douglas NJ. The effect of clustered versus regular sleep fragmentation on daytime function. J Sleep Res. 1999;8:305–311. doi: 10.1046/j.1365-2869.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- Martin SE, Engleman HM, Deary IJ, Douglas NJ. The effect of sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1996;153:1328–1332. doi: 10.1164/ajrccm.153.4.8616562. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci USA. 2006;103:19170–175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–454. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Ra SM, Kim H, Jang MH, Shin MC, Lee TH, Lim BV, Kim CJ, Kim EH, Kim KM, Kim SS. Treadmill running and swimming increase cell proliferation in the hippocampal dentate gyrus of rats. Neurosci Lett. 2002;333:123–6. doi: 10.1016/s0304-3940(02)01031-5. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Roman V, Van der Borght K, Leemburg SA, Van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- Stepanski E, Lamphere J, Roehrs T, Zorick F, Roth T. Experimental sleep fragmentation in normal subjects. Int J Neurosci. 1987;33:207–214. doi: 10.3109/00207458708987405. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15:815–21. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134:721–3. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- van der Borght K, Ferrari F, Klauke K, Roman V, Havekes R, Sgoifo A, van der Zee EA, Meerlo Hippocampla cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav Brain Res. 2006;167:36–41. doi: 10.1016/j.bbr.2005.08.012. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pragg H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]