Abstract
This study measured cell proliferation in the hippocampal dentate gyrus in the adult rat at different times within a 12:12 h light-dark cycle. The experiments were conducted in animals living in either a complex environment or in standard lab cages. A single dose of the thymidine analog 5-Bromo-2′–deoxyuridine (BrdU) was injected 2 h before animals were sacrificed either 4, 11, 16, or 23 hours after the beginning of the light phase of the light/dark cycle (designated ZT0). In both studies, we found a significant increase in the number of BrdU-positive cells in the subgranular cell layer (SGZ) following BrdU administration at ZT 9 and sacrifice at ZT11, compared to other circadian times examined. BrdU administration at ZT9 was timed to primarily identify proliferating cells that were in the S phase of the cell cycle during the light phase. Our results suggest that cell proliferation is enhanced either by sleep or by other variables coupled to the light phase of the circadian cycle.
Keywords: Cell proliferation, dentate gyrus, hippocampus, circadian, BrdU
Introduction
The proliferation and maturation of new neurons in the dentate gyrus (DG) in adult mammals has been shown to play a role in certain hippocampal-dependent cognitive capabilities [13]. For this reason, the identification of physiological and experiential processes that act as positive or negative modulators of cell proliferation and maturation is of importance. Sustained sleep deprivation and sleep fragmentation have been shown to be among the potent negative modulators of proliferation [5, 7]. However, there have been two interpretations of these findings. On one hand, it has been argued that sleep-related processes have pro-proliferative effects, related to other anabolic and plasticity-related functions associated with sleep [6]. Alternatively, it has been argued that application of sleep deprivation procedures may be stressful, and that the effects of sleep deprivation can be explained as in terms of stress [15]. One type of data that is relevant to this issue in the distribution proliferation within the 24 h light/dark cycle. Since the rat and mouse species used for most studies have more sleep occurring during the light phase of the 24-hour day, a hypothesis that sleep has pro-proliferative effects leads to a prediction that more proliferation would occur in the light phase. However, three studies have provided evidence that there is no circadian pattern of proliferation [1, 9, 17] and a fourth found increased proliferation during the light phase only in the hilus of the DG [10]. We have re-examined this issue, considering that only one previous study was done in rats, which were used in most sleep deprivation studies. We initially studied rats in which we attempted to reinforce circadian rhythmicity by providing an enriched environment to stimulate nocturnal activity, and places to provide dark shelters during the light. When we observed circadian rhythmicity in proliferation under these conditions, we also studied standard cage conditions.
Methods
All protocols were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Animal Care and Use Committee at the V.A.G.L.A.H.S.
Male Sprague-Dawley rats of approximately 2 months of age (~300g) were used in this study. Animals were kept in pairs in a sound proof chamber at 23° C under artificial 12-h light (rest period) and 12-h dark (activity period) cycle with lights on at 6 AM (designated ZT0, zeitgeber time, in accordance with circadian conventions), and with food and water available ad libitum. These conditions were maintained for 2 weeks before the experiments were performed, in order to ensure that the animals were entrained to the light-dark cycle. The cages where animals where housed were serviced at the beginning of the dark period.
Experiment 1
Light-dark cycle variations of cell proliferation in rats living in enriched environment. A total of 20 animals were used in this experiment. Rats, 2 per cage, were housed in a large cage (54×58×60cm). This cage was arranged with nesting material, plastic tubes, which provided dark nest sites, and soft plastic toys. A new toy was introduced in the cage at the beginning of the dark period every day for 7 days. Standard rat chow was supplemented with wheat-based cereal (fruit loops). The thymidine analog, 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich, 300 mg/kg) was injected i.p. 2 h before animals were sacrificed either 4, 11, 16, or 23 hours after the beginning of the light phase.
Experiment 2
Light-dark cycle differences in cell proliferation in rats living in standard cages. A total of 19 rats were used in this experiment. In this experiment animals were housed, 2 per cage, in Plexiglas cages (27×29×30cm) without enrichment. Standard food was introduced at the beginning of the dark period and animals were sacrificed at ZT11 and ZT23, 2 hrs after administration of BrdU (300 mg/Kg).
Subjects from all groups were deeply anesthetized (Nembutal 100 mg/kg), perfused transcardially with PB 0.1 M followed by ice cold paraformaldehyde (4%); brains were removed and stored in 10% and 30% sucrose at 4°C until they sank. Brains were cut in 40 μm coronal sections. Sections encompassing the hippocampus were preserved in a cryoprotectant solution containing sucrose, polyvinyl-pyrrolidone (PVP-40, Sigma) and ethylene glycol dissolved in PB pH 7.2, which provides long-term protection of the tissue. Sections were processed for BrdU immunohistochemistry.
To visualize the expression of the BrdU we used the peroxidase method (ABC system, Vectastain, Vector Laboratories). Immunohistochemistry was performed simultaneously on sections from different time points to maximize the reliability of comparisons across groups. Staining was performed on slide-mounted sections. A one-in-six series of sections was pretreated for BrdU immunostaining by DNA denaturation (2 M HCl at 37 °C for 30 min) followed by 10 min in borate buffer (pH 8.5). Tissue was rinsed in TBS 0.1M. Sections were then incubated with a mouse anti-BrdU (BD Biosciences 1:400) primary antibody for 48 h. Tissue from both groups was treated with aliquots from the same batch of antibodies. Sections were subsequently incubated with a biotinylated horse anti-mouse IgG (1:200, Vector Laboratories), then reacted with avidin–biotin complex (1:100, Vector Elite) and developed with diaminobenzidine tetrahydrochloride (DAB, Sigma). Absence of the primary antibody resulted in an absence of specific nuclear staining.
BrdU-positive cells were counted using a 40x objective (Nikon E600) throughout the rostrocaudal extent of the DG granule cell layer. The optical fractionator method was used for counting as previously described [5]. Stereo Investigator software was used to estimate the total number of BrdU positive cells per DG by utilizing the optical fractionator formula.
To study distribution of newborn cells, their numbers were counted separately per hippocampal sub regions, i.e. the granule cell layer (GCL)/subgranular zone (SGZ) and the hilus. The SGZ was defined as a two cell thick layer along the inner border of the GCL and the hilus.
Differences in cell counts between groups were assessed with one-way ANOVA followed by Fisher LSD post-hoc test or Students’ t-test for a 2-group comparison. A P value of < 0.05 was adopted for significance.
Results
Experiment 1
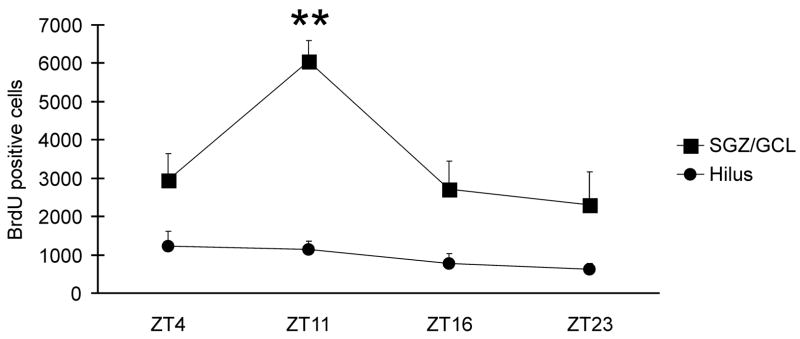
We first quantified the number of labeled cells within SGZ/GCL following BrdU exposure at different circadian times in rats living in an enriched environment. The number of BrdU+ cells significantly varied across circadian times (F (3,16)=6.3, P<0.01, one-way ANOVA). As it is shown in Fig. 1, cell proliferation was the highest in animals sacrificed at ZT11 (the end of the light phase) and at its minimum in animals sacrificed at ZT23 (the end of the dark phase). At ZT11 the number of BrdU+ cells was significantly higher than at the rest of circadian times tested (P=0.001 for ZT23, P< 0.01 for ZT16 and P=0.01 for ZT 4, Fisher LSD test). The remaining pairwise comparisons did not reveal statistically significant differences.
Fig. 1.
In rats living in an enriched environment, the numbers of proliferating cells in the SGZ and GCL identified by BrdU-labeling was dependent on the time of BrdU administration within the light-dark cycle. Labeled cell counts were significantly higher after BrdU administration at ZT9 and animals were sacrificed at ZT11, at the end of the light phase of the light-dark cycle. There was no significant effect of circadian time on labeling in the hilus. ** P < 0.01.
In the hilus (Fig. 1), the numbers of BrdU+ cells were not significantly different among the circadian times examined (F(3,16) =1.6, P = 0.2).
Experiment 2
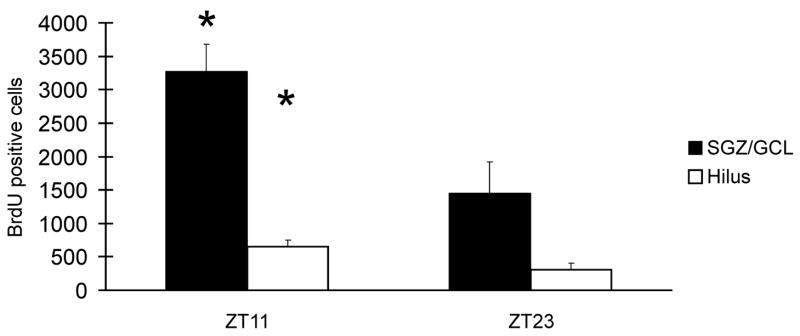
We quantified the rate of proliferation in standard cage conditions after BrdU exposure at two time points, ZT11 and ZT23, where the highest and lowest number of BrdU+ cells were seen under enriched conditions (Fig. 2). We found a higher number of BrdU+ cells at ZT11 in both the GCL/SGZ (P < 0.01, t = 3.0, df=17) and the hilus (P < 0.05, t = 2.3, df=17).
Fig. 2.
In rats living in standard cage conditions, the numbers of proliferating cells identified by BrdU labeling were significantly higher in both SGZ/GLC and hilus when animals were injected with BrdU at ZT9 and sacrificed at ZT11 compared to ZT21/23 schedule. * P < 0.05.
Discussion
Our study was designed to measure proliferation at times of maximum and minimum amounts of sleep within the 24 h period, considering the concept that BrdU labels proliferating cells in the S-phase, which is estimated to last 9 hrs in the adult rat, and BrdU exposure after administration is limited to 2 hrs [3]. BrdU is available for uptake by cells in the S phase of the cell cycle for 2 hours following IP administration [16]. Moreover, recently it has been shown that minimum time of BrdU availability for incorporation into DNA is less than 15 min [14]. Thus, BrdU-labeled cell counts obtained after administration at ZT9 and sacrifice at ZT11 identifies proliferation during the first 11 hrs of the light cycle, and BrdU administration at ZT21 and sacrifice at ZT23 identifies proliferation during the dark phase. In contrast to previous studies, our studies showed significantly higher proliferation in animals sacrificed at ZT11 under both standard and enriched caging conditions. This finding is congruent with the hypothesis that proliferation is increased either during sleep or by other circadian variables associated with the light phase. Potential stress-inducing factors were minimized.
In the first experiment we found a peak in proliferation only when BrdU was administered at ZT9 (sacrifice at ZT11), although BrdU administration at ZT2 or ZT14 would also identify proliferation during parts of the light phase. This finding suggests that proliferation may peak during a restricted part of the light phase, but further work is needed to confirm this possibility. Circadian variations in the bioavailability of BrdU for uptake by proliferating cells after IP administration have not been described, but we cannot rule out this possibility.
Several factors could account for the differences between the present study and previous studies. Three of the four studies used previously were conducted in mice [9, 10, 17]. Either species differences or other methodological details could be important. The S-phase of the cell cycle duration has been shown to be shorter in mice [8] than in rats [3]. Thus, single BrdU injections will sample a more limited part of the circadian cycle. In one mouse study, there was increased proliferation during the light phase in the hilus of the DG [10], a finding replicated in our standard housing study. Another mouse study was based on labeling by the endogenous marker of cell proliferation, Ki-67 which labels all phases of the cell cycle except G0 [17]. As the mouse DG proliferating cell cycle duration is 14 hr [14], this marker may fail to reveal shorter-term variations in proliferation, as noted previously [10]. Another mouse study [9] was based on daily BrdU injections for 7 consecutive days before sacrifice, so proliferation counts could be influenced by factors affecting survival of proliferating cells over the 7 day study period. The rat study [1] also found no significant circadian variation in DG cell proliferation. However, this study used a 24 h survival time post administration of BrdU, which makes the cohort of proliferating cells susceptible to other influences in the period after BrdU injection.
We also note that in the present study servicing was done and new food was introduced into the cages at the beginning of the dark period. It has been shown that food presentation can alter circadian entrainment in rats [11]. Food availability was not restricted in our study.
Circadian rhythmicity in proliferation may result from effects of circadian gene expression on the cell cycle. The expression of the circadian gene, Per2, is strongly expressed in the DG [12], and, was reported to have permissive inhibitory role on cell proliferation [4]. Indeed, it has been reported that increases in cell proliferation in the DG were coincident with reductions in Per2 expression in this region [2]. In adult rats the lowest point in the circadian variation of expression of Per2 in the DG occurs at ZT10 [12], about the time we found maximal proliferation.
Our study has methodological implications for studies measuring proliferation in the DG. First, measurement of the rate of proliferation is strongly dependent on the timing of BrdU administration within the circadian cycle. Researchers typically carefully control the timing of light-dark exposure of experimental groups and match the circadian timing of sacrifice of experimental groups. However, animal servicing procedures or experimental manipulations that differentially alter experimental and control groups with respect to entrainment to the light-dark cycle could affect the outcome of experiments.
Acknowledgments
We gratefully acknowledge Feng Xu and Keng-Tee Chew for their excellent assistance. This research was made possible by a grant from the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine to RGM. Supported by the US Department of Veterans Affairs Medical Research service and US National Institutes of Health grants NIMH 075076, HL 60296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–72. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- 2.Borgs L, Malgrange B, Nguyen L, Hans G, Mangin J, Moonen G, Maquet P, Albrecht U, Belachew S. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. The circadian gene Per2 controls progenitor cell proliferation and neurogenesis in the adult hippocampus Program No. 607.1. [Google Scholar]
- 3.Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 4.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plyas an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Marin R, Suntsova N, Stewart DR, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–71. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman-Marin R, McGinty D. Sleep deprivation suppresses adult neurogenesis: clues to the role of sleep in brain plasticity. Sleep and Biological Rhythms. 2006;4:27–34. [Google Scholar]
- 7.Guzman-Marin R, Bashir RT, Suntsova N, Szymusiak R, McGinty D. Adult hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.05.030. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes NL, Nowarkowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 9.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 10.Kochman LJ, Weber ET, Fornal FA, Jacobs BL. Circadian variation in mouse hippocampal cell proliferation. Neurosci Lett. 2006;406:256–259. doi: 10.1016/j.neulet.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Krieger DT. Houser H Comparison of synchronization of circadian corticosteroid rhythms by photoperiod and food. Proc Natl Acad Sci USA. 1978;75:1577–81. doi: 10.1073/pnas.75.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A 2005. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lledo PM, Alonso M, Grubb M. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 14.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci USA. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowakowski RS, Lwein SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 17.Van der Borght K, Ferrari F, Klauke K, Roman V, Havekes R, Sgoifo A, van der Zee EA, Meerlo P. Hippocampal cell proliferation across the day: increase by running wheel activity, but no effect of sleep and wakefulness. Behav Brain Res. 2006;167:36–41. doi: 10.1016/j.bbr.2005.08.012. [DOI] [PubMed] [Google Scholar]