Abstract
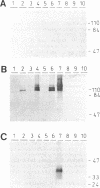
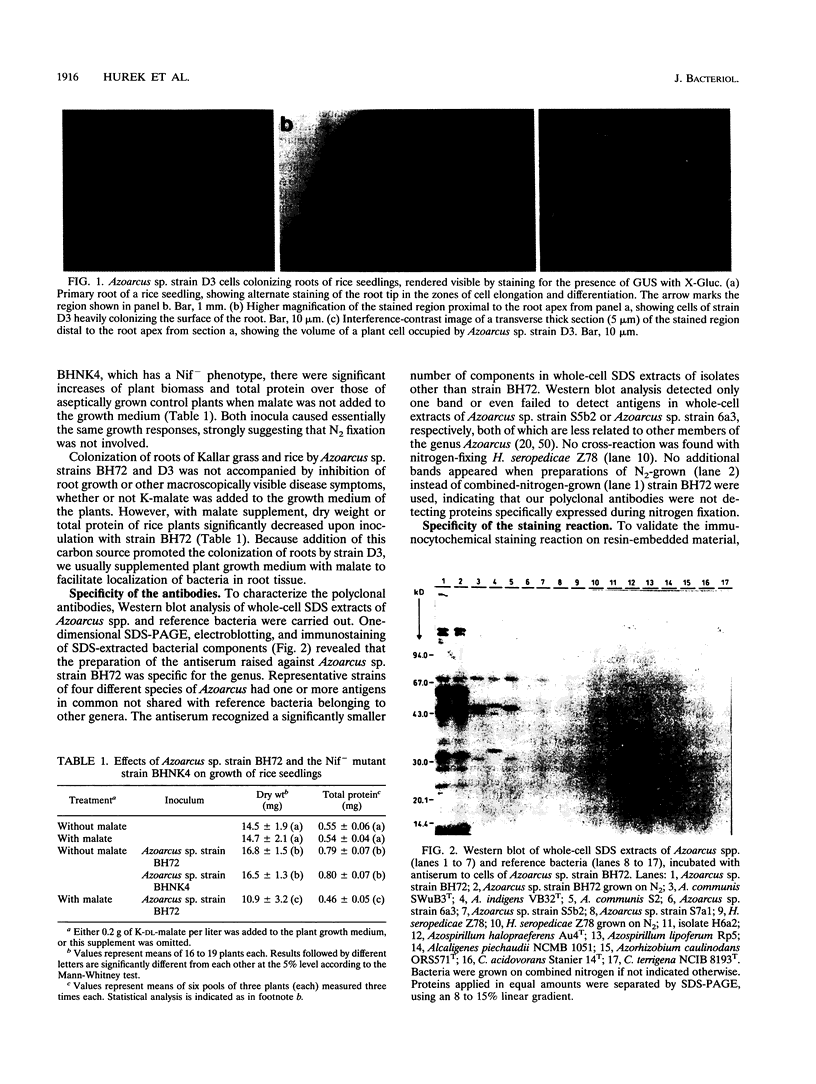
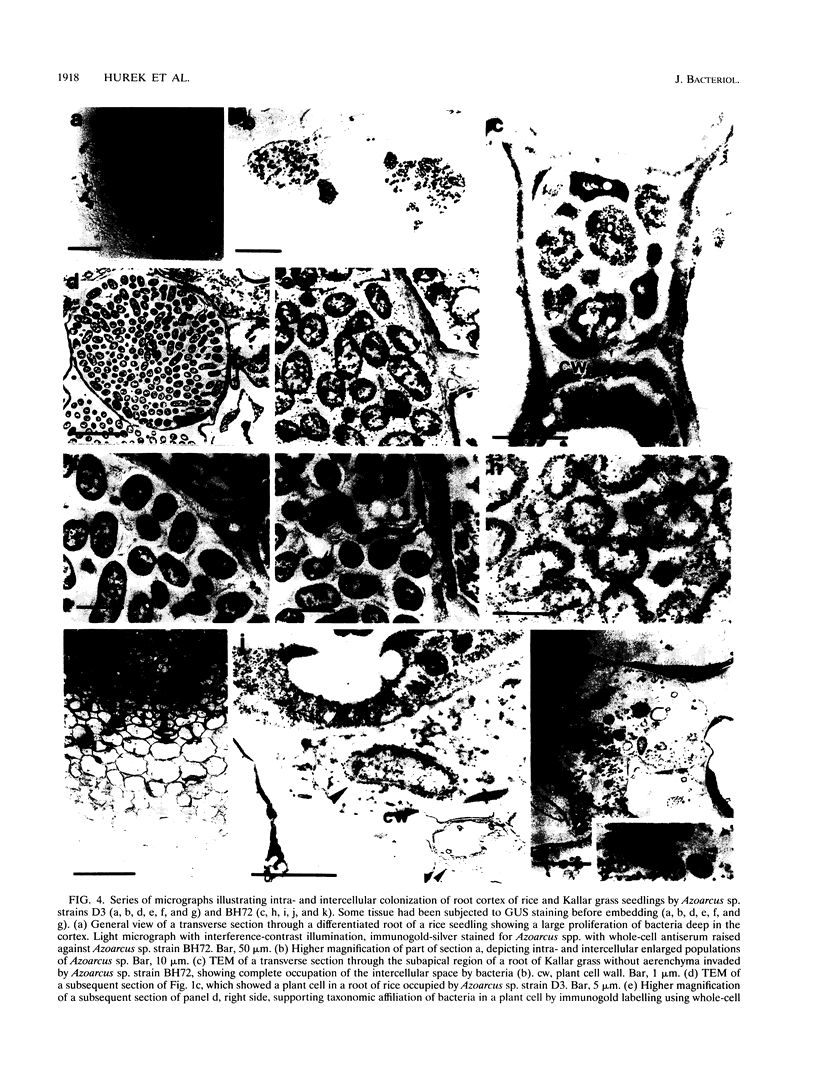
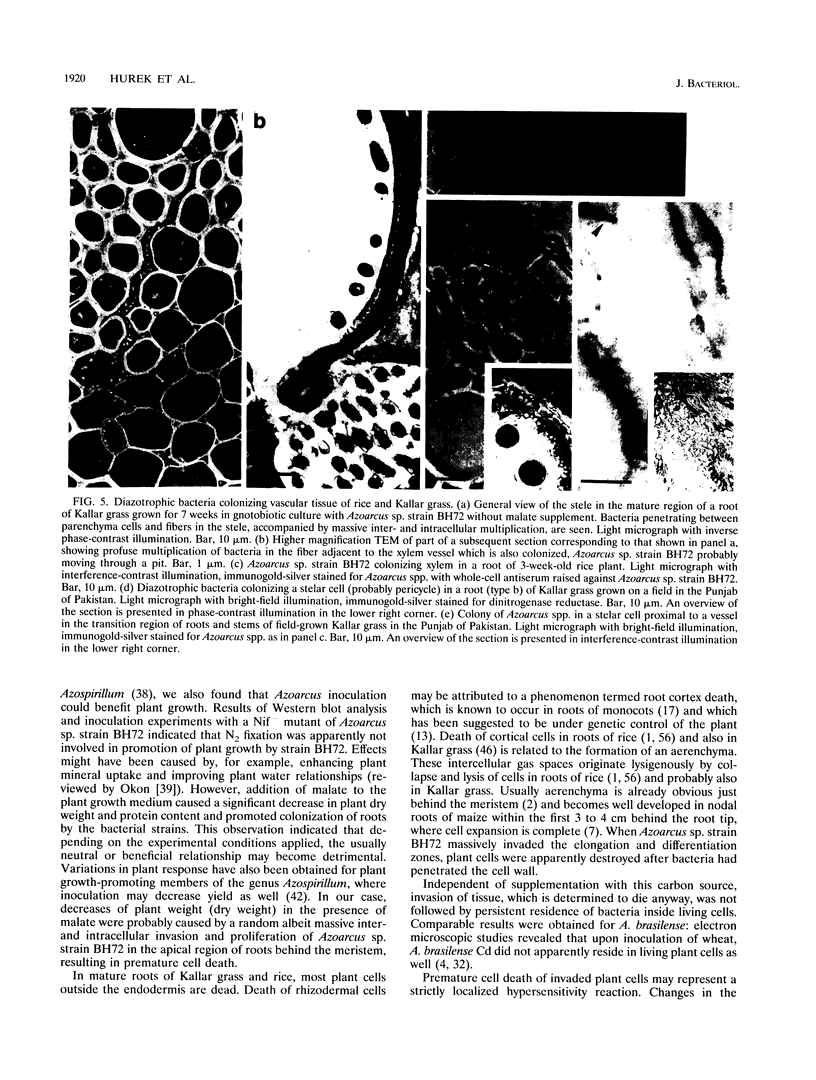
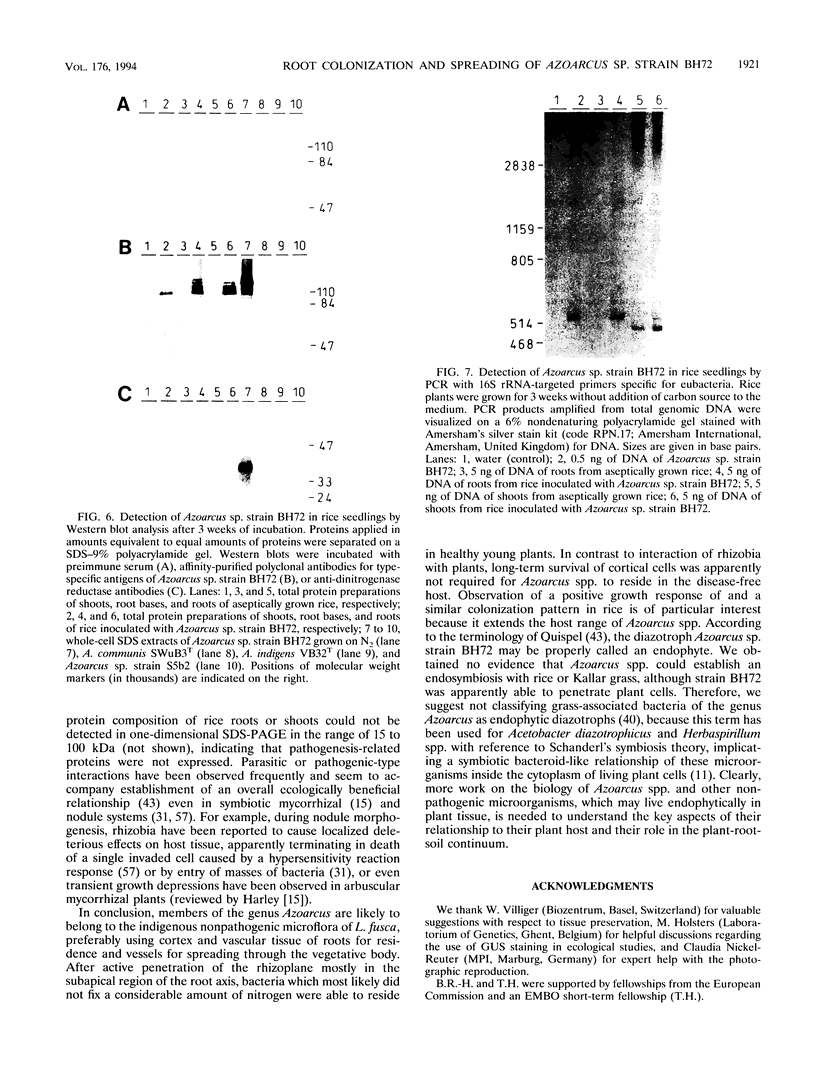
The invasive properties of Azoarcus sp. strain BH72, an endorhizospheric isolate of Kallar grass, on gnotobiotically grown seedlings of Oryza sativa IR36 and Leptochloa fusca (L.) Kunth were studied. Additionally, Azoarcus spp. were localized in roots of field-grown Kallar grass. To facilitate localization and to assure identity of bacteria, genetically engineered microorganisms expressing beta-glucuronidase were also used as inocula. beta-Glucuronidase staining indicated that the apical region of the root behind the meristem was the most intensively colonized. Light and electron microscopy showed that strain BH72 penetrated the rhizoplane preferentially in the zones of elongation and differentiation and colonized the root interior inter- and intracellularly. In addition to the root cortex, stelar tissue was also colonized; bacteria were found in the xylem. No evidence was obtained that Azoarcus spp. could reside in living plant cells; rather, plant cells were apparently destroyed after bacteria had penetrated the cell wall. A common pathogenicity test on tobacco leaves provided no evidence that representative strains of Azoarcus spp. are phytopathogenic. Compared with the control, inoculation with strain BH72 significantly promoted growth of rice seedlings. This effect was reversed when the plant medium was supplemented with malate (0.2 g/liter). N2 fixation was apparently not involved, because the same response was obtained with a nifK mutant of strain BH72, which has a Nif- phenotype. Also, Western blot (immunoblot) analysis of protein extracts from rice seedlings gave no indication that nitrogenase was present. PCR and Western immunoblotting, using primers specific for eubacteria and antibodies recognizing type-specific antigens, respectively, indicated that strain BH72 could colonize rice plants systemically, probably mediated by longitudinal spreading through vessels.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hurek T., Burggraf S., Woese C. R., Reinhold-Hurek B. 16S rRNA-targeted polymerase chain reaction and oligonucleotide hybridization to screen for Azoarcus spp., grass-associated diazotrophs. Appl Environ Microbiol. 1993 Nov;59(11):3816–3824. doi: 10.1128/aem.59.11.3816-3824.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurek T., Reinhold B., Fendrik I., Niemann E. G. Root-Zone-Specific Oxygen Tolerance of Azospirillum spp. and Diazotrophic Rods Closely Associated with Kallar Grass. Appl Environ Microbiol. 1987 Jan;53(1):163–169. doi: 10.1128/aem.53.1.163-169.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEMENT Z. RAPID DETECTION OF THE PATHOGENICITY OF PHYTOPATHOGENIC PSEUDOMONADS. Nature. 1963 Jul 20;199:299–300. doi: 10.1038/199299b0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucocq J. M., Roth J. Applications of immunocolloids in light microscopy. III. Demonstration of antigenic and lectin-binding sites in semithin resin sections. J Histochem Cytochem. 1984 Oct;32(10):1075–1083. doi: 10.1177/32.10.6384362. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin D. G., Döbereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can J Microbiol. 1978 Jun;24(6):734–742. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B., Hurek T., Claeyssens M., van Montagu M. Cloning, expression in Escherichia coli, and characterization of cellulolytic enzymes of Azoarcus sp., a root-invading diazotroph. J Bacteriol. 1993 Nov;175(21):7056–7065. doi: 10.1128/jb.175.21.7056-7065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Cross-reaction of predominant nitrogen-fixing bacteria with enveloped, round bodies in the root interior of kallar grass. Appl Environ Microbiol. 1987 Apr;53(4):889–891. doi: 10.1128/aem.53.4.889-891.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Niemann E. G., Fendrik I. Close association of azospirillum and diazotrophic rods with different root zones of kallar grass. Appl Environ Microbiol. 1986 Sep;52(3):520–526. doi: 10.1128/aem.52.3.520-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. B., Signer E. R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 1990 Mar;4(3):344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch K. A., Bradley D. J., Knox J. P., Perotto S., Butcher G. W., Brewin N. J. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989 Feb;8(2):335–341. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]