Abstract
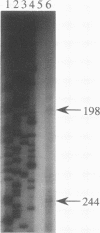
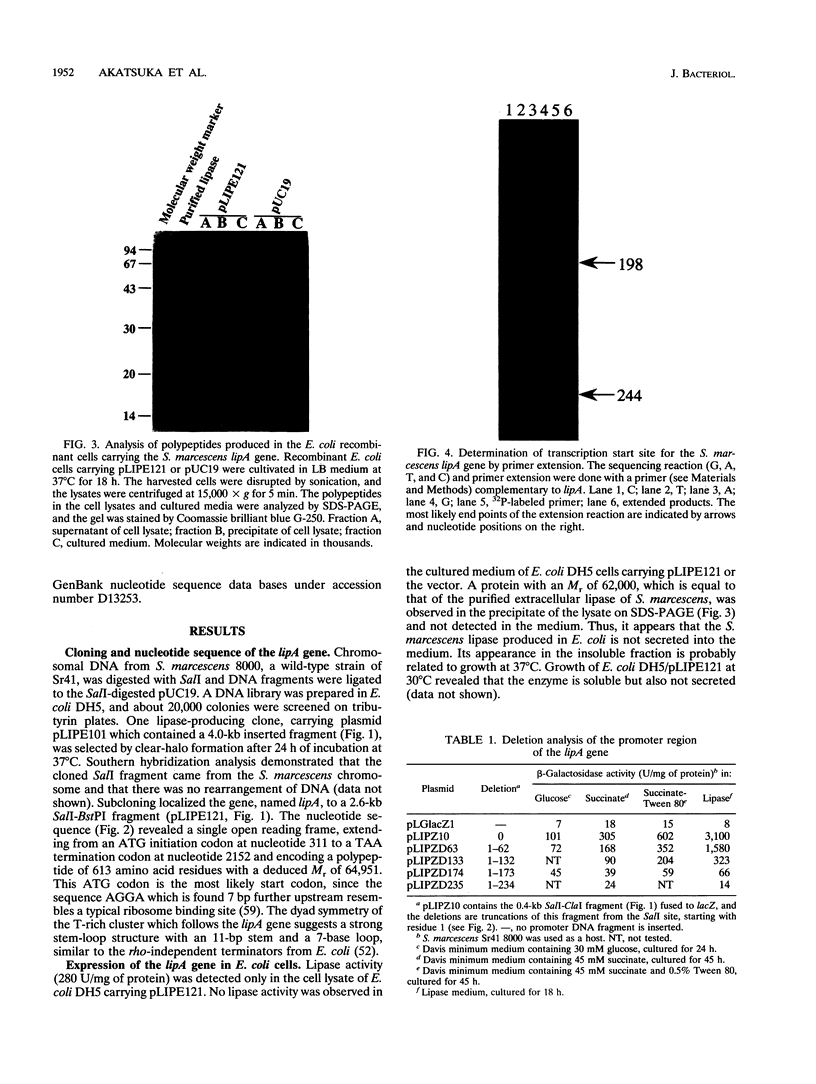
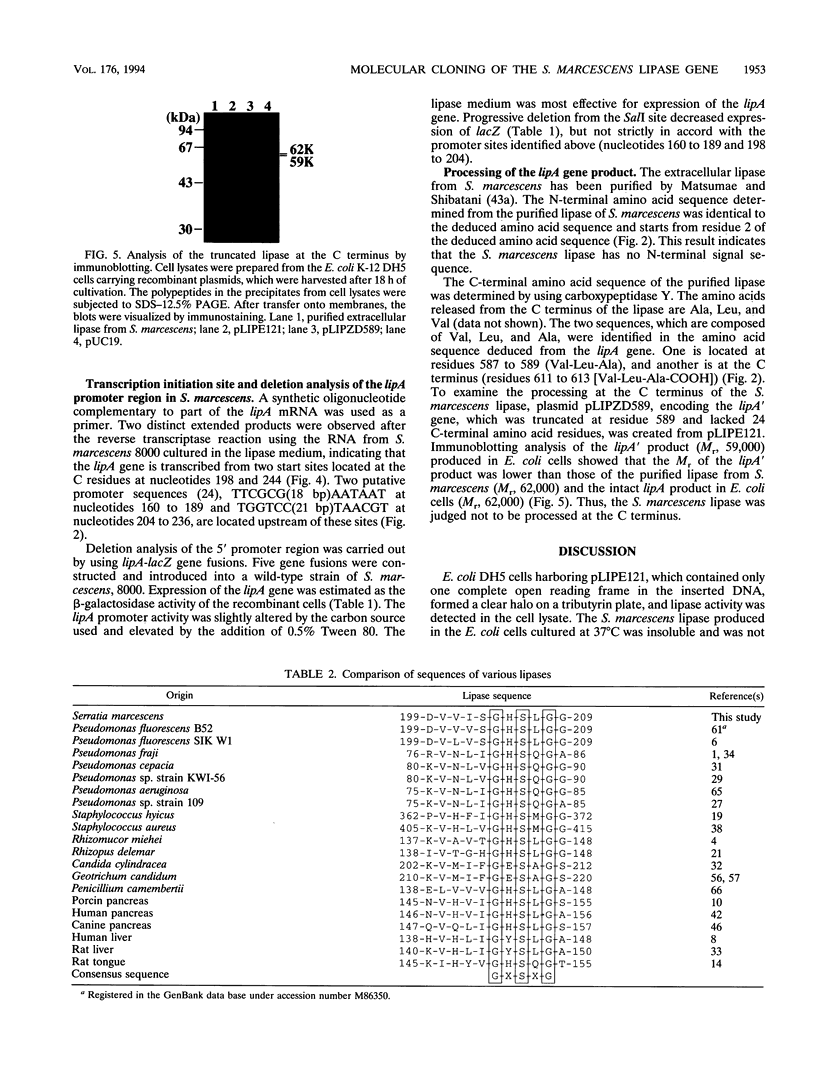
The lipA gene encoding an extracellular lipase was cloned from the wild-type strain of Serratia marcescens Sr41. Nucleotide sequencing showed a major open reading frame encoding a 64.9-kDa protein of 613 amino acid residues; the deduced amino acid sequence contains a lipase consensus sequence, GXSXG. The lipase had 66 and 56% homologies with the lipases of Pseudomonas fluorescens B52 and P. fluorescens SIK W1, respectively, but did not show any overall homology with lipases from other origins. The Escherichia coli cells carrying the S. marcescens lipA gene did not secrete the lipase into the medium. The S. marcescens lipase had no conventional N-terminal signal sequence but was also not subjected to any processing at both the N-terminal and C-terminal regions. A specific short region similar to the regions of secretory proteins having no N-terminal signal peptide was observed in the amino acid sequence. Expression of the lipA gene in S. marcescens was affected by the carbon source and the addition of Tween 80.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama S., Yoshida N., Inouye S. Cloning, sequencing and expression of the lipase gene from Pseudomonas fragi IFO-12049 in E. coli. FEBS Lett. 1988 Dec 19;242(1):36–40. doi: 10.1016/0014-5793(88)80980-3. [DOI] [PubMed] [Google Scholar]
- Ball T. K., Saurugger P. N., Benedik M. J. The extracellular nuclease gene of Serratia marcescens and its secretion from Escherichia coli. Gene. 1987;57(2-3):183–192. doi: 10.1016/0378-1119(87)90121-1. [DOI] [PubMed] [Google Scholar]
- Blight M. A., Holland I. B. Structure and function of haemolysin B,P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990 Jun;4(6):873–880. doi: 10.1111/j.1365-2958.1990.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Boel E., Huge-Jensen B., Christensen M., Thim L., Fiil N. P. Rhizomucor miehei triglyceride lipase is synthesized as a precursor. Lipids. 1988 Jul;23(7):701–706. doi: 10.1007/BF02535672. [DOI] [PubMed] [Google Scholar]
- Brady L., Brzozowski A. M., Derewenda Z. S., Dodson E., Dodson G., Tolley S., Turkenburg J. P., Christiansen L., Huge-Jensen B., Norskov L. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature. 1990 Feb 22;343(6260):767–770. doi: 10.1038/343767a0. [DOI] [PubMed] [Google Scholar]
- Chung G. H., Lee Y. P., Jeohn G. H., Yoo O. J., Rhee J. S. Cloning and nucleotide sequence of thermostable lipase gene from Pseudomonas fluorescens SIK W1. Agric Biol Chem. 1991 Sep;55(9):2359–2365. [PubMed] [Google Scholar]
- Datta S., Luo C. C., Li W. H., VanTuinen P., Ledbetter D. H., Brown M. A., Chen S. H., Liu S. W., Chan L. Human hepatic lipase. Cloned cDNA sequence, restriction fragment length polymorphisms, chromosomal localization, and evolutionary relationships with lipoprotein lipase and pancreatic lipase. J Biol Chem. 1988 Jan 25;263(3):1107–1110. [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi. Proteases B and C are synthesized and secreted as zymogens without a signal peptide. J Biol Chem. 1989 May 25;264(15):9083–9089. [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protein secretion in gram-negative bacteria. The extracellular metalloprotease B from Erwinia chrysanthemi contains a C-terminal secretion signal analogous to that of Escherichia coli alpha-hemolysin. J Biol Chem. 1990 Oct 5;265(28):17118–17125. [PubMed] [Google Scholar]
- Docherty A. J., Bodmer M. W., Angal S., Verger R., Riviere C., Lowe P. A., Lyons A., Emtage J. S., Harris T. J. Molecular cloning and nucleotide sequence of rat lingual lipase cDNA. Nucleic Acids Res. 1985 Mar 25;13(6):1891–1903. doi: 10.1093/nar/13.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A., Hamilton W. D., Johnston A. W., Downie J. A. The Rhizobium nodulation gene nodO encodes a Ca2(+)-binding protein that is exported without N-terminal cleavage and is homologous to haemolysin and related proteins. EMBO J. 1990 Feb;9(2):349–354. doi: 10.1002/j.1460-2075.1990.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givskov M., Olsen L., Molin S. Cloning and expression in Escherichia coli of the gene for extracellular phospholipase A1 from Serratia liquefaciens. J Bacteriol. 1988 Dec;170(12):5855–5862. doi: 10.1128/jb.170.12.5855-5862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J., Duong F., Wandersman C., Murgier M., Lazdunski A. The secretion genes of Pseudomonas aeruginosa alkaline protease are functionally related to those of Erwinia chrysanthemi proteases and Escherichia coli alpha-haemolysin. Mol Microbiol. 1991 Feb;5(2):447–453. doi: 10.1111/j.1365-2958.1991.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Götz F., Popp F., Korn E., Schleifer K. H. Complete nucleotide sequence of the lipase gene from Staphylococcus hyicus cloned in Staphylococcus carnosus. Nucleic Acids Res. 1985 Aug 26;13(16):5895–5906. doi: 10.1093/nar/13.16.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Allen J., Berka T. R. Cloning, expression and characterization of a cDNA encoding a lipase from Rhizopus delemar. Gene. 1991 Dec 20;109(1):107–113. doi: 10.1016/0378-1119(91)90594-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hobson A. H., Buckley C. M., Aamand J. L., Jørgensen S. T., Diderichsen B., McConnell D. J. Activation of a bacterial lipase by its chaperone. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5682–5686. doi: 10.1073/pnas.90.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara F., Kageyama Y., Hirata M., Nihira T., Yamada Y. Purification, characterization, and molecular cloning of lactonizing lipase from Pseudomonas species. J Biol Chem. 1991 Sep 25;266(27):18135–18140. [PubMed] [Google Scholar]
- Iizumi T., Nakamura K., Shimada Y., Sugihara A., Tominaga Y., Fukase T. Cloning, nucleotide sequencing, and expression in Escherichia coli of a lipase and its activator genes from Pseudomonas sp. KWI-56. Agric Biol Chem. 1991 Sep;55(9):2349–2357. [PubMed] [Google Scholar]
- Jones J. D., Grady K. L., Suslow T. V., Bedbrook J. R. Isolation and characterization of genes encoding two chitinase enzymes from Serratia marcescens. EMBO J. 1986 Mar;5(3):467–473. doi: 10.1002/j.1460-2075.1986.tb04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen S., Skov K. W., Diderichsen B. Cloning, sequence, and expression of a lipase gene from Pseudomonas cepacia: lipase production in heterologous hosts requires two Pseudomonas genes. J Bacteriol. 1991 Jan;173(2):559–567. doi: 10.1128/jb.173.2.559-567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Honda H., Taniguchi-Morimura J., Iwasaki S. The codon CUG is read as serine in an asporogenic yeast Candida cylindracea. Nature. 1989 Sep 14;341(6238):164–166. doi: 10.1038/341164a0. [DOI] [PubMed] [Google Scholar]
- Komaromy M. C., Schotz M. C. Cloning of rat hepatic lipase cDNA: evidence for a lipase gene family. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1526–1530. doi: 10.1073/pnas.84.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugimiya W., Otani Y., Hashimoto Y., Takagi Y. Molecular cloning and nucleotide sequence of the lipase gene from Pseudomonas fragi. Biochem Biophys Res Commun. 1986 Nov 26;141(1):185–190. doi: 10.1016/s0006-291x(86)80352-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kurooka S., Okamoto S., Hashimoto M. A novel and simple colorimetric assay for human serum lipase. J Biochem. 1977 Feb;81(2):361–369. doi: 10.1093/oxfordjournals.jbchem.a131467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. E., Rosenblum J. L., Strauss A. W. Cloning and characterization of human pancreatic lipase cDNA. J Biol Chem. 1989 Nov 25;264(33):20042–20048. [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Cloning and expression in Escherichia coli of the Serratia marcescens metalloprotease gene: secretion of the protease from E. coli in the presence of the Erwinia chrysanthemi protease secretion functions. J Bacteriol. 1991 Apr;173(7):2160–2166. doi: 10.1128/jb.173.7.2160-2166.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Mickel F. S., Weidenbach F., Swarovsky B., LaForge K. S., Scheele G. A. Structure of the canine pancreatic lipase gene. J Biol Chem. 1989 Aug 5;264(22):12895–12901. [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K., Suzuki S., Imai Y., Komatsubara S. Analysis of the Serratia marcescens proBA operon and feedback control of proline biosynthesis. J Gen Microbiol. 1991 Mar;137(3):509–517. doi: 10.1099/00221287-137-3-509. [DOI] [PubMed] [Google Scholar]
- Peoples O. P., Liebl W., Bodis M., Maeng P. J., Follettie M. T., Archer J. A., Sinskey A. J. Nucleotide sequence and fine structural analysis of the Corynebacterium glutamicum hom-thrB operon. Mol Microbiol. 1988 Jan;2(1):63–72. doi: 10.1111/j.1365-2958.1988.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu A. K., Economou A., Hong G. F., Ghelani S., Johnston A. W., Downie J. A. Secretion of the Rhizobium leguminosarum nodulation protein NodO by haemolysin-type systems. Mol Microbiol. 1992 Jan;6(2):231–238. doi: 10.1111/j.1365-2958.1992.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Schrag J. D., Li Y. G., Wu S., Cygler M. Ser-His-Glu triad forms the catalytic site of the lipase from Geotrichum candidum. Nature. 1991 Jun 27;351(6329):761–764. doi: 10.1038/351761a0. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Sugihara A., Iizumi T., Tominaga Y. cDNA cloning and characterization of Geotrichum candidum lipase II. J Biochem. 1990 May;107(5):703–707. doi: 10.1093/oxfordjournals.jbchem.a123112. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Sugihara A., Tominaga Y., Iizumi T., Tsunasawa S. cDNA molecular cloning of Geotrichum candidum lipase. J Biochem. 1989 Sep;106(3):383–388. doi: 10.1093/oxfordjournals.jbchem.a122862. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Kisumi M. Isolation of a versatile Serratia marcescens mutant as a host and molecular cloning of the aspartase gene. J Bacteriol. 1985 Jan;161(1):1–6. doi: 10.1128/jb.161.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y., Miller K. J. Cloning, expression, and nucleotide sequence of a lipase gene from Pseudomonas fluorescens B52. Appl Environ Microbiol. 1992 Apr;58(4):1402–1407. doi: 10.1128/aem.58.4.1402-1407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler U. K., Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979 Jun;138(3):663–670. doi: 10.1128/jb.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth S., Hoesche C., Strunk C., Winkler U. K. Molecular genetics of the extracellular lipase of Pseudomonas aeruginosa PAO1. J Gen Microbiol. 1992 Jul;138(7):1325–1335. doi: 10.1099/00221287-138-7-1325. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Mase T., Takeuchi K. Cloning and structure of the mono- and diacylglycerol lipase-encoding gene from Penicillium camembertii U-150. Gene. 1991 Jul 15;103(1):61–67. doi: 10.1016/0378-1119(91)90391-n. [DOI] [PubMed] [Google Scholar]
- Yanagida N., Uozumi T., Beppu T. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):937–944. doi: 10.1128/jb.166.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Caro J., Boudouard M., Bonicel J., Guidoni A., Desnuelle P., Rovery M. Porcine pancreatic lipase. Completion of the primary structure. Biochim Biophys Acta. 1981 Dec 29;671(2):129–138. doi: 10.1016/0005-2795(81)90126-4. [DOI] [PubMed] [Google Scholar]
- de la Peña P., Zasloff M. Enhancement of mRNA nuclear transport by promoter elements. Cell. 1987 Aug 14;50(4):613–619. doi: 10.1016/0092-8674(87)90034-1. [DOI] [PubMed] [Google Scholar]