Abstract
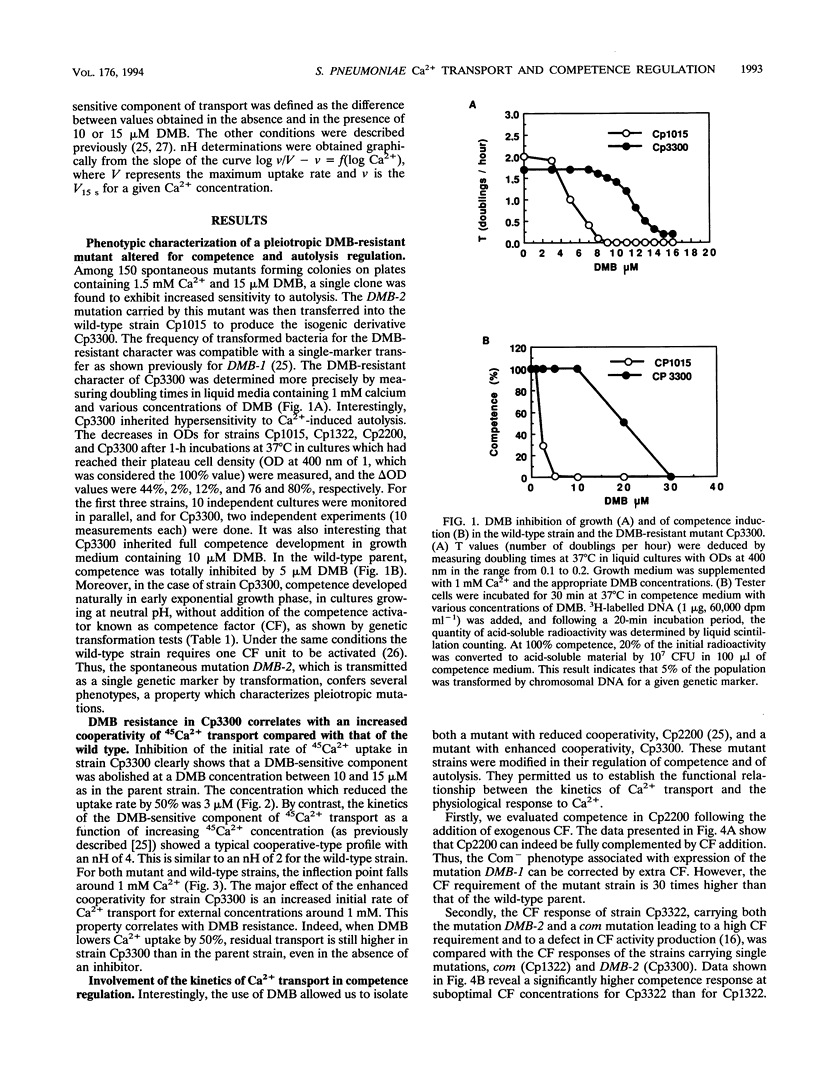
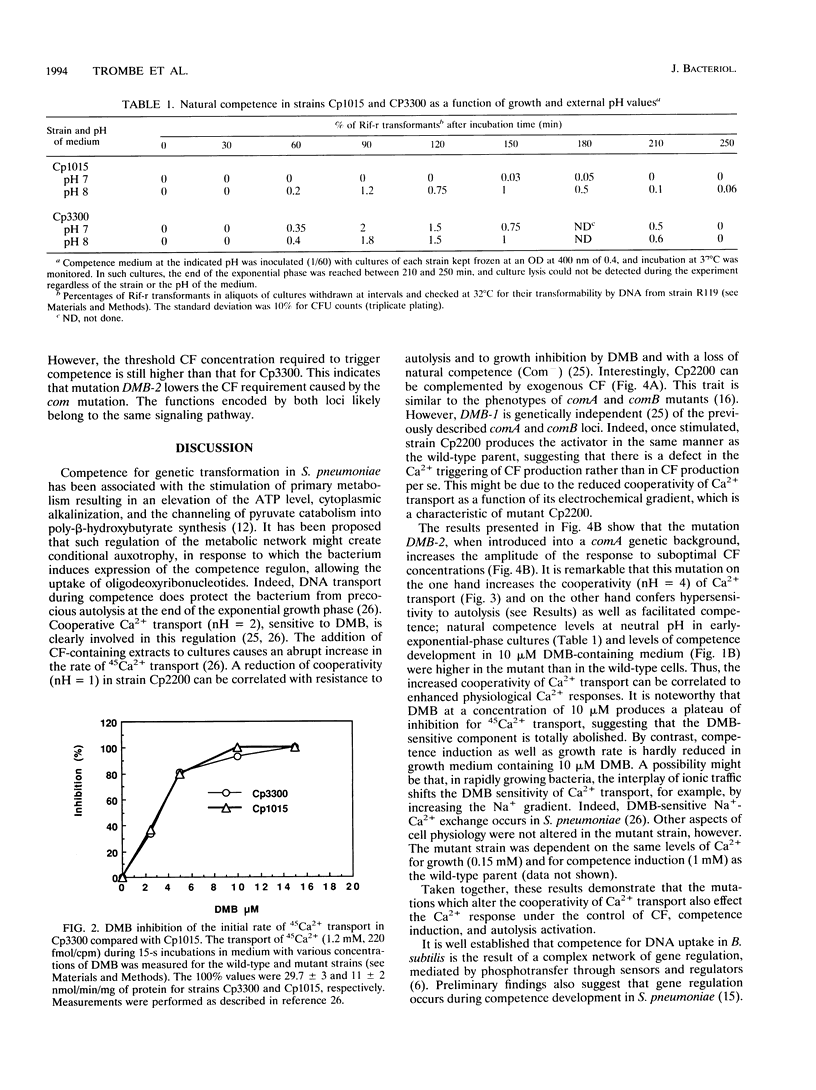
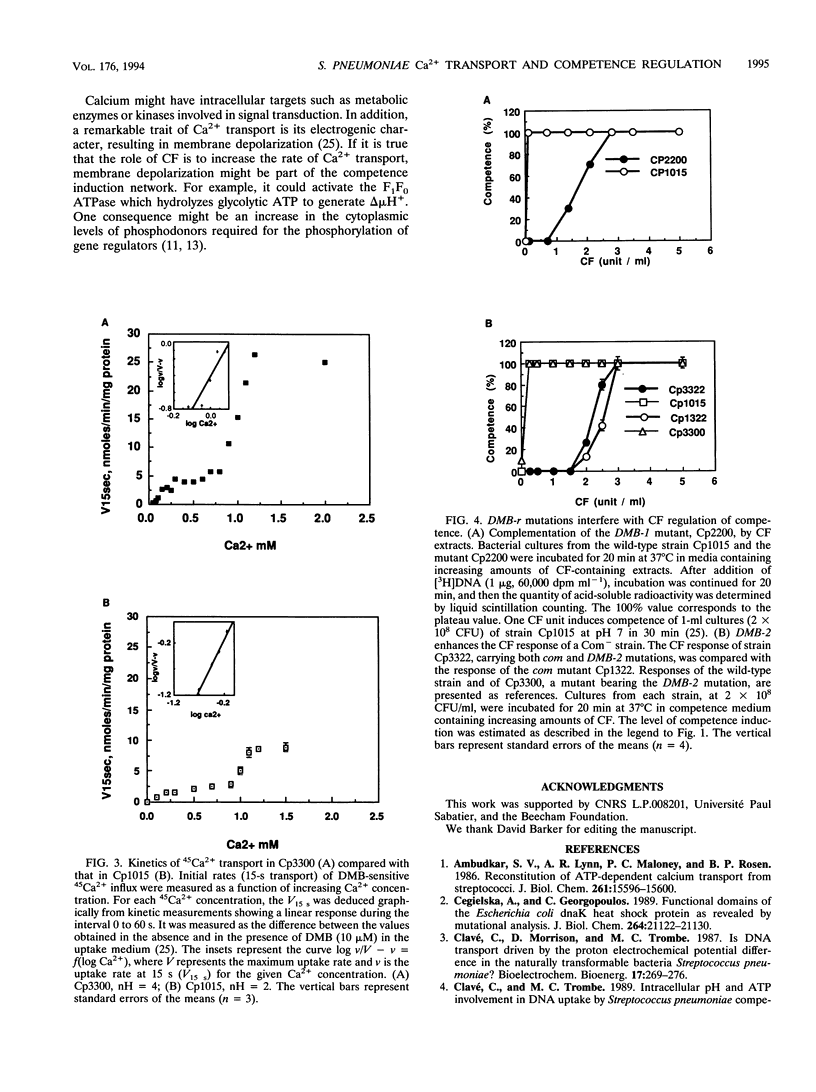
In Streptococcus pneumoniae, Ca2+ induces a stress response which is regulated by a proteic activator known as competence factor (CF). This stress response is expressed as the induction of competence for DNA uptake and genetic transformation in exponentially growing cultures and by autolysis in late exponential phase. DNA transport during competence can be described as a homeostatic response that prevents autolysis of the cultures. Electrogenic and cooperative calcium transport with a Hill number (nH) of 2 appears to mediate this Ca2+ response. Mutant strains altered in their kinetics for Ca2+ transport, with nHs of 1 and 4, were isolated and characterized in order to address the role of the kinetics of Ca2+ transport in the Ca2+ response. The reduced cooperativity of Ca2+ uptake in mutant strain Cp2200 was associated with an absolute requirement for added CF to develop competence and with resistance to autolysis. The enhanced cooperativity of Ca2+ uptake in mutant strain Cp3300 was associated with facilitated competence and hypersensitivity to autolysis. Moreover, the mutation carried by strain Cp3300 increases the CF response of previously described competence-defective mutants. The pleiotropic mutants Cp2200 and Cp3300 allowed us to demonstrate that cooperativity of transport determines the Ca2+ response in S. pneumoniae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Lynn A. R., Maloney P. C., Rosen B. P. Reconstitution of ATP-dependent calcium transport from streptococci. J Biol Chem. 1986 Nov 25;261(33):15596–15600. [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991 Sep;55(3):395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Initiation of bacterial transformation. Nature. 1957 Jun 29;179(4574):1322–1325. doi: 10.1038/1791322a0. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Hughes C., Frey J. Functional analysis of the Ca(2+)-regulated hemolysin I operon of Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1992 Aug;60(8):3059–3064. doi: 10.1128/iai.60.8.3059-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Van Brunt J., Harold F. M. ATP-linked calcium transport in cells and membrane vesicles of Streptococcus faecalis. J Biol Chem. 1978 Apr 10;253(7):2085–2092. [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lee T. Y., Makino K., Shinagawa H., Nakata A. Overproduction of acetate kinase activates the phosphate regulon in the absence of the phoR and phoM functions in Escherichia coli. J Bacteriol. 1990 May;172(5):2245–2249. doi: 10.1128/jb.172.5.2245-2249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A., Clavé C., Capeyrou R., Lafontan V., Trombe M. C. Ionic and energetic changes at competence in the naturally transformable bacterium Streptococcus pneumoniae. J Gen Microbiol. 1989 Aug;135(8):2189–2197. doi: 10.1099/00221287-135-8-2189. [DOI] [PubMed] [Google Scholar]
- Lukat G. S., McCleary W. R., Stock A. M., Stock J. B. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T., Hirata H., Kusaka I. Calcium channel blockers inhibit bacterial chemotaxis. FEBS Lett. 1988 Aug 29;236(2):437–440. doi: 10.1016/0014-5793(88)80072-3. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Baker M. F. Competence for genetic transformation in pneumococcus depends on synthesis of a small set of proteins. Nature. 1979 Nov 8;282(5735):215–217. doi: 10.1038/282215a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Trombe M. C., Hayden M. K., Waszak G. A., Chen J. D. Isolation of transformation-deficient Streptococcus pneumoniae mutants defective in control of competence, using insertion-duplication mutagenesis with the erythromycin resistance determinant of pAM beta 1. J Bacteriol. 1984 Sep;159(3):870–876. doi: 10.1128/jb.159.3.870-876.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris V., Chen M., Goldberg M., Voskuil J., McGurk G., Holland I. B. Calcium in bacteria: a solution to which problem? Mol Microbiol. 1991 Apr;5(4):775–778. doi: 10.1111/j.1365-2958.1991.tb00748.x. [DOI] [PubMed] [Google Scholar]
- O'Hara M. B., Hageman J. H. Energy and calcium ion dependence of proteolysis during sporulation of Bacillus subtilis cells. J Bacteriol. 1990 Aug;172(8):4161–4170. doi: 10.1128/jb.172.8.4161-4170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onek L. A., Smith R. J. Calmodulin and calcium mediated regulation in prokaryotes. J Gen Microbiol. 1992 Jun;138(6):1039–1049. doi: 10.1099/00221287-138-6-1039. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Calcium ion regulates chemotactic behaviour in bacteria. Nature. 1977 Nov 3;270(5632):66–67. doi: 10.1038/270066a0. [DOI] [PubMed] [Google Scholar]
- Rampersaud A., Utsumi R., Delgado J., Forst S. A., Inouye M. Ca2(+)-enhanced phosphorylation of a chimeric protein kinase involved with bacterial signal transduction. J Biol Chem. 1991 Apr 25;266(12):7633–7637. [PubMed] [Google Scholar]
- Seto H., Tomasz A. Calcium-requiring step in the uptake of deoxyribonucleic acid molecules through the surface of competent pneumococci. J Bacteriol. 1976 Jun;126(3):1113–1118. doi: 10.1128/jb.126.3.1113-1118.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa L. S., Adler J. Calcium ions are involved in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11804–11808. doi: 10.1073/pnas.89.24.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa L. S., Olivera B. M., Adler J. Inhibition of Escherichia coli chemotaxis by omega-conotoxin, a calcium ion channel blocker. J Bacteriol. 1993 Mar;175(5):1235–1238. doi: 10.1128/jb.175.5.1235-1238.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombe M. C. Characterization of a calcium porter of Streptococcus pneumoniae involved in calcium regulation of growth and competence. J Gen Microbiol. 1993 Mar;139(3):433–439. doi: 10.1099/00221287-139-3-433. [DOI] [PubMed] [Google Scholar]
- Trombe M. C., Clavé C., Manias J. M. Calcium regulation of growth and differentiation in Streptococcus pneumoniae. J Gen Microbiol. 1992 Jan;138(1):77–84. doi: 10.1099/00221287-138-1-77. [DOI] [PubMed] [Google Scholar]
- Trombe M. C., Lanéelle G., Sicard A. M. Characterization of a Streptococcus pneumoniae mutant with altered electric transmembrane potential. J Bacteriol. 1984 Jun;158(3):1109–1114. doi: 10.1128/jb.158.3.1109-1114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij W., Bulthuis R., Postma E., Konings W. N. Calcium transport in membrane vesicles of Bacillus subtilis. J Bacteriol. 1985 Dec;164(3):1294–1300. doi: 10.1128/jb.164.3.1294-1300.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



