Abstract
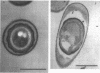
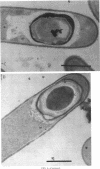
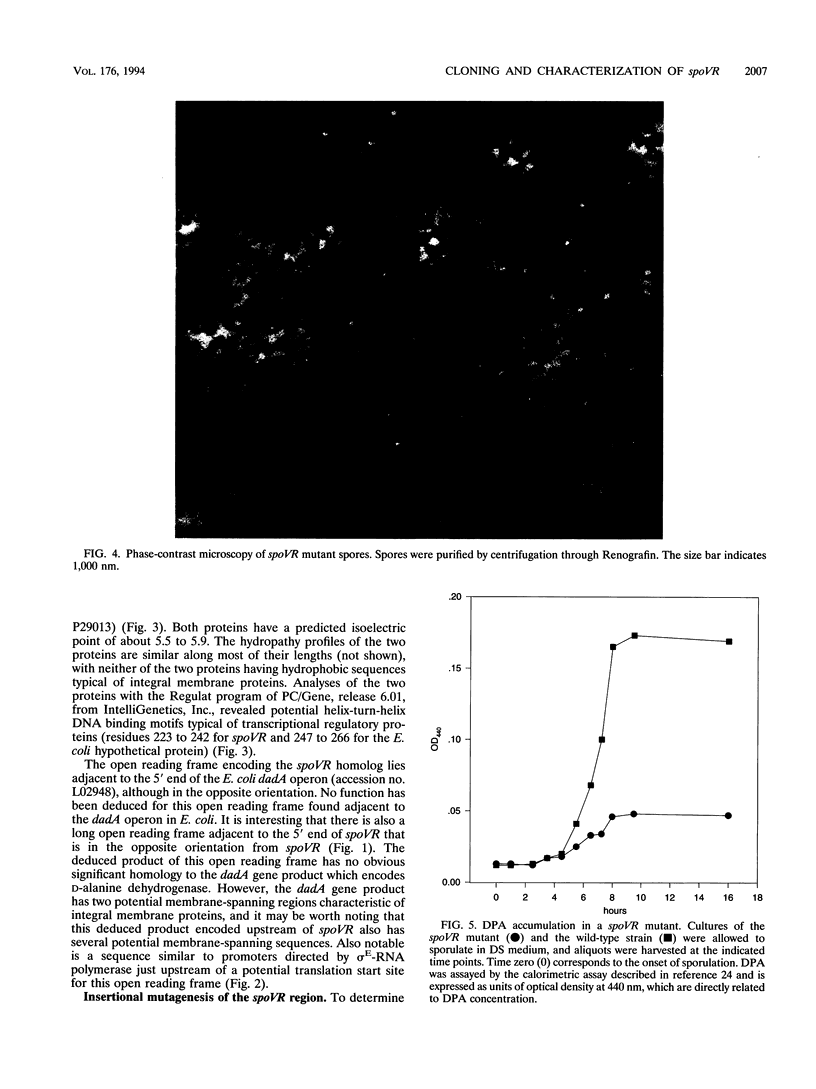
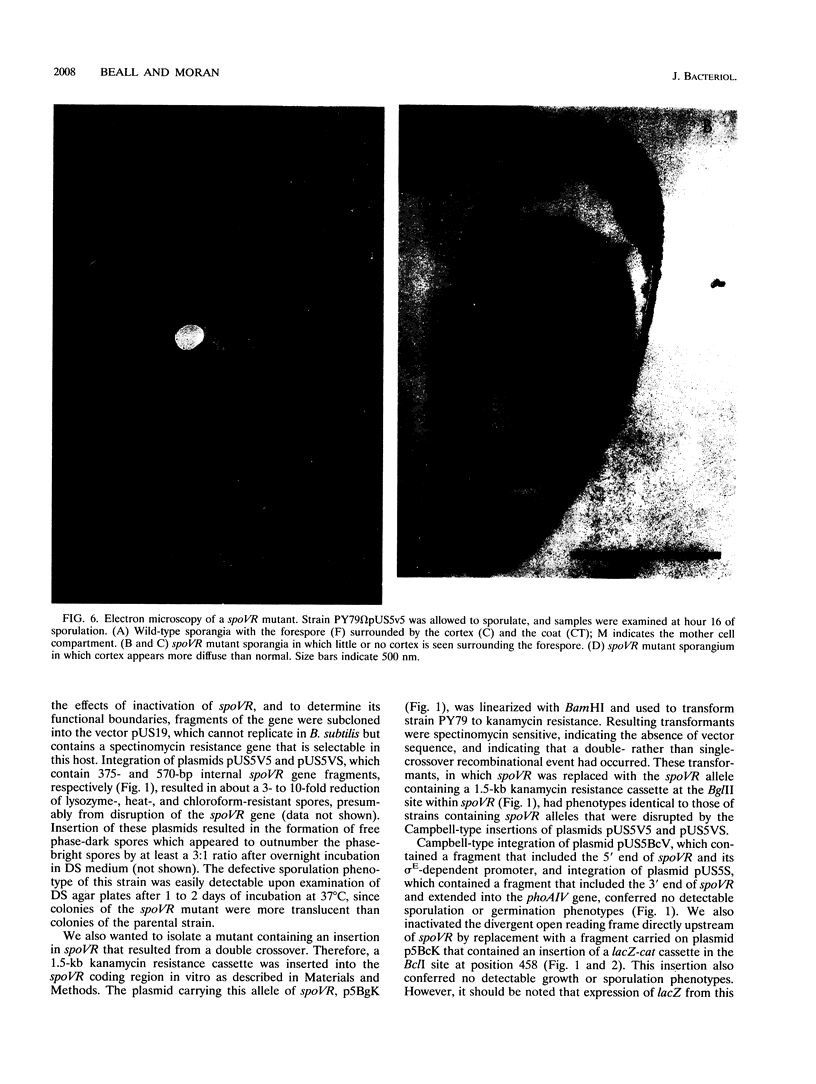
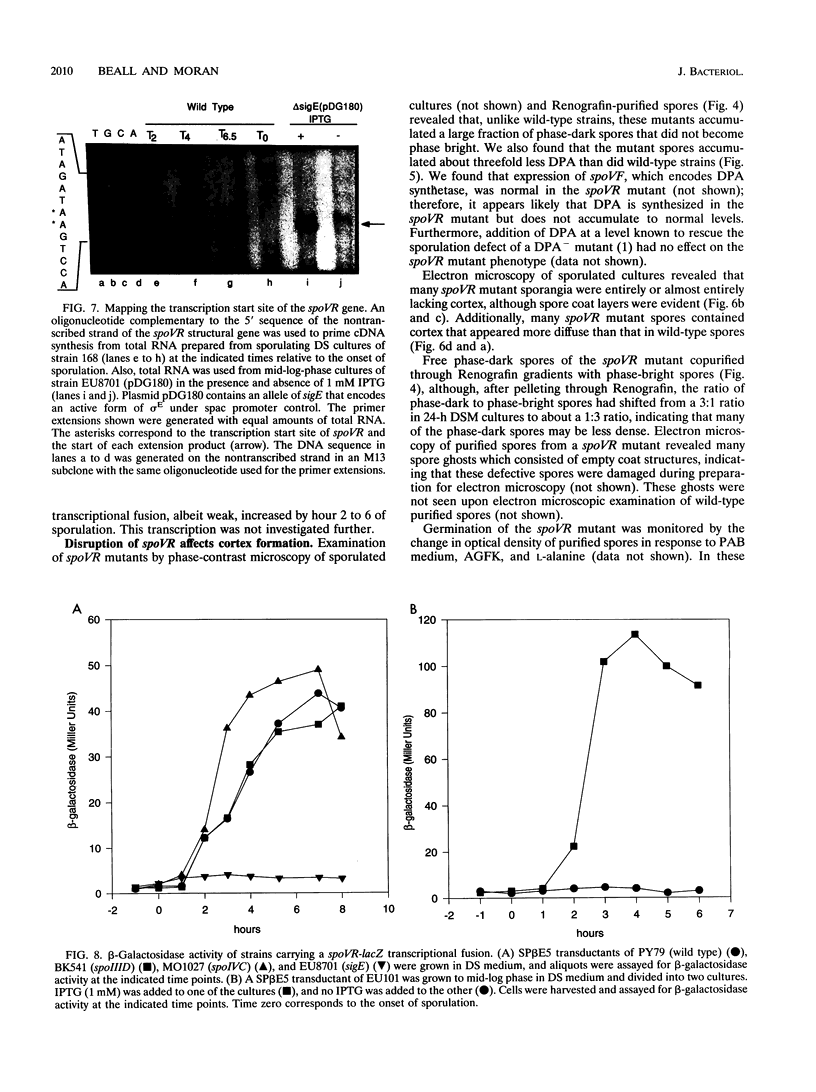
Screening for sigma E-dependent promoters led to the isolation of a gene from Bacillus subtilis, designated spoVR, which appears to be involved in spore cortex formation. Cultures of strains carrying mutations in spoVR had an increased proportion of phase-dark spores, which correlated with an increased proportion of cortexless spores seen by electron microscopy. The numbers of heat- and chloroform-resistant phase-bright spores produced by these mutants were decreased by about 3- to 10-fold, and accumulation of dipicolinate was decreased by more than 3-fold. The spoVR gene was located on the B. subtilis chromosome immediately upstream from, and in the opposite orientation of, the phoAIV gene. Expression of spoVR was initiated at the second hour of sporulation from a sigma E-dependent promoter, and this expression did not require any of the other known mother-cell-specific transcriptional regulators. The spoVR gene was predicted to encode a product of 468 residues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balassa G., Milhaud P., Raulet E., Silva M. T., Sousa J. C. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J Gen Microbiol. 1979 Feb;110(2):365–379. doi: 10.1099/00221287-110-2-365. [DOI] [PubMed] [Google Scholar]
- Beall B., Driks A., Losick R., Moran C. P., Jr Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat. J Bacteriol. 1993 Mar;175(6):1705–1716. doi: 10.1128/jb.175.6.1705-1716.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Ling M. L. Isolation and sequence analysis of dacB, which encodes a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992 Mar;174(6):1717–1725. doi: 10.1128/jb.174.6.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- Daniel R. A., Drake S., Buchanan C. E., Scholle R., Errington J. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J Mol Biol. 1994 Jan 7;235(1):209–220. doi: 10.1016/s0022-2836(05)80027-0. [DOI] [PubMed] [Google Scholar]
- Daniel R. A., Errington J. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis. J Mol Biol. 1993 Jul 20;232(2):468–483. doi: 10.1006/jmbi.1993.1403. [DOI] [PubMed] [Google Scholar]
- Diederich B., Tatti K. M., Jones C. H., Beall B., Moran C. P., Jr Genetic suppression analysis of sigma E interaction with three promoters in sporulating Bacillus subtilis. Gene. 1992 Nov 2;121(1):63–69. doi: 10.1016/0378-1119(92)90162-i. [DOI] [PubMed] [Google Scholar]
- Driks A., Roels S., Beall B., Moran C. P., Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 1994 Jan;8(2):234–244. doi: 10.1101/gad.8.2.234. [DOI] [PubMed] [Google Scholar]
- Errington J. A general method for fusion of the Escherichia coli lacZ gene to chromosomal genes in Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2953–2966. doi: 10.1099/00221287-132-11-2953. [DOI] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques A. O., de Lencastre H., Piggot P. J. A Bacillus subtilis morphogene cluster that includes spoVE is homologous to the mra region of Escherichia coli. Biochimie. 1992 Jul-Aug;74(7-8):735–748. doi: 10.1016/0300-9084(92)90146-6. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., Kim E. E., Bookstein C., Kapp N. V., Edwards C. W., Wyckoff H. W. Bacillus subtilis alkaline phosphatases III and IV. Cloning, sequencing, and comparisons of deduced amino acid sequence with Escherichia coli alkaline phosphatase three-dimensional structure. J Biol Chem. 1991 Jan 15;266(2):1077–1084. [PubMed] [Google Scholar]
- Illing N., Errington J. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma E and sigma F in prespore engulfment. J Bacteriol. 1991 May;173(10):3159–3169. doi: 10.1128/jb.173.10.3159-3169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imae Y., Strominger J. L. Relationship between cortex content and properties of Bacillus sphaericus spores. J Bacteriol. 1976 May;126(2):907–913. doi: 10.1128/jb.126.2.907-913.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp N. V., Edwards C. W., Chesnut R. S., Hulett F. M. The Bacillus subtilis phoAIV gene: effects of in vitro inactivation on total alkaline phosphatase production. Gene. 1990 Nov 30;96(1):95–100. doi: 10.1016/0378-1119(90)90346-s. [DOI] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel B., Kroos L., Poth H., Youngman P., Losick R. Temporal and spatial control of the mother-cell regulatory gene spoIIID of Bacillus subtilis. Genes Dev. 1989 Nov;3(11):1735–1744. doi: 10.1101/gad.3.11.1735. [DOI] [PubMed] [Google Scholar]
- Lazarevic V., Margot P., Soldo B., Karamata D. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-L-alanine amidase and its modifier. J Gen Microbiol. 1992 Sep;138(9):1949–1961. doi: 10.1099/00221287-138-9-1949. [DOI] [PubMed] [Google Scholar]
- Levin P. A., Fan N., Ricca E., Driks A., Losick R., Cutting S. An unusually small gene required for sporulation by Bacillus subtilis. Mol Microbiol. 1993 Aug;9(4):761–771. doi: 10.1111/j.1365-2958.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Stragier P. Cloning, characterization, and expression of the spoVB gene of Bacillus subtilis. J Bacteriol. 1991 Dec;173(24):7942–7949. doi: 10.1128/jb.173.24.7942-7949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Coppolecchia R., DeGrazia H., Moran C. P., Jr Negative regulator of sigma G-controlled gene expression in stationary-phase Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):709–715. doi: 10.1128/jb.172.2.709-715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Rosenkrantz M. S., Sonenshein A. L. Transcriptional control of the Bacillus subtilis spoIID gene. J Bacteriol. 1986 Mar;165(3):771–779. doi: 10.1128/jb.165.3.771-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. M., Errington J. Differential gene expression during sporulation in Bacillus subtilis: structure and regulation of the spoIIID gene. Mol Microbiol. 1990 Apr;4(4):543–551. doi: 10.1111/j.1365-2958.1990.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Jones C. H., Moran C. P., Jr Genetic evidence for interaction of sigma E with the spoIIID promoter in Bacillus subtilis. J Bacteriol. 1991 Dec;173(24):7828–7833. doi: 10.1128/jb.173.24.7828-7833.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeragool G., Miyao A., Yamada K., Sato T., Kobayashi Y. In vivo expression of the Bacillus subtilis spoVE gene. J Bacteriol. 1993 Jul;175(13):4071–4080. doi: 10.1128/jb.175.13.4071-4080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L. B., Donovan W. P., Fitz-James P. C., Losick R. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 1988 Aug;2(8):1047–1054. doi: 10.1101/gad.2.8.1047. [DOI] [PubMed] [Google Scholar]
- Zheng L. B., Losick R. Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol. 1990 Apr 20;212(4):645–660. doi: 10.1016/0022-2836(90)90227-d. [DOI] [PubMed] [Google Scholar]